Abstract
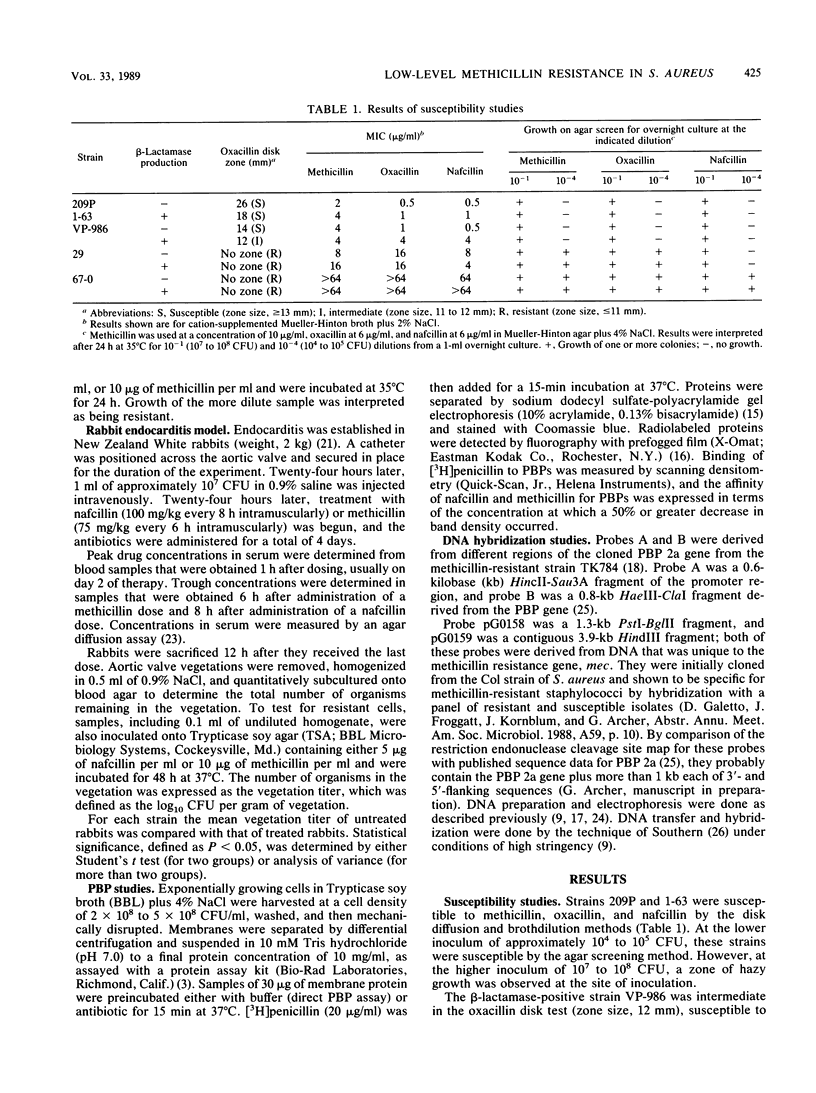
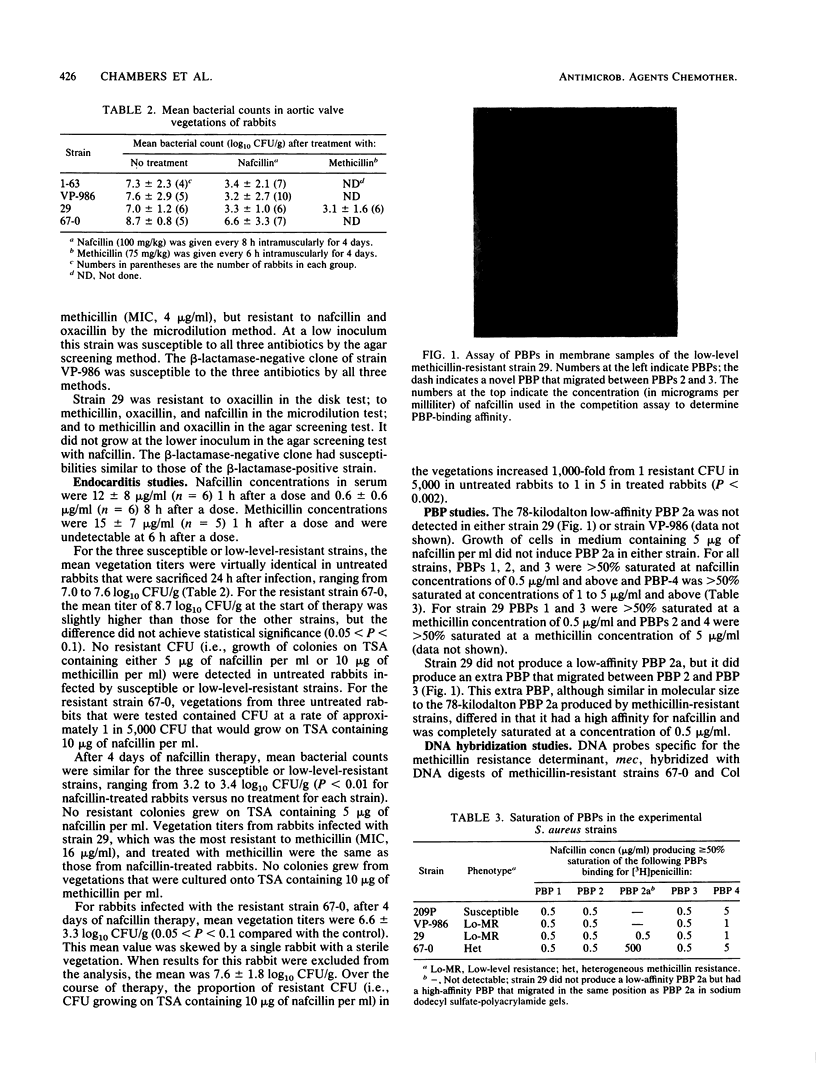
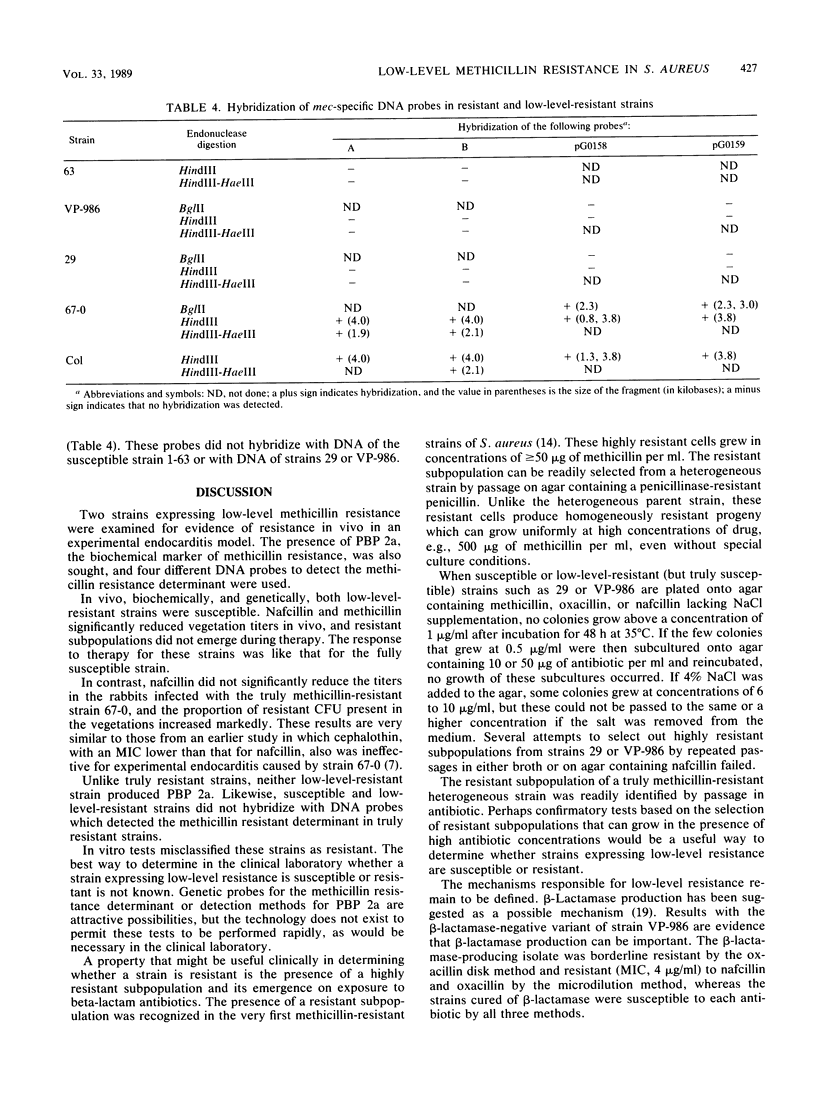
Two strains of Staphylococcus aureus expressing borderline or low-level methicillin resistance by one or more in vitro test methods were examined for resistance in vivo and for biochemical and genetic markers of methicillin resistance. In vivo, nafcillin was equally effective against experimental aortic valve endocarditis in rabbits, regardless of whether they were infected by a fully susceptible or a low-level-resistant strain. Resistance did not emerge during therapy. For the more resistant of the two low-level-resistant strains, methicillin was as effective as nafcillin. Nafcillin was ineffective against endocarditis caused by a truly methicillin-resistant strain, and resistance emerged on therapy. The low-level-resistant strains did not produce the low-affinity penicillin-binding protein 2a that is associated with methicillin resistance and did not contain DNA that hybridized with probes that recognized the methicillin resistance determinant. Low-level resistance in S. aureus is a phenomenon that is biochemically and genetically distinct from true methicillin resistance. These strains actually are susceptible to beta-lactam antibiotics. The clinical problem posed by these strains is not a therapeutic one but, instead, one of how to differentiate them from those that are truly methicillin resistant.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER M. Methicillin-resistant staphylococci. J Clin Pathol. 1961 Jul;14:385–393. doi: 10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Bruns W., Keppeler H., Baucks R. Suppression of intrinsic resistance to penicillins in Staphylococcus aureus by polidocanol, a dodecyl polyethyleneoxid ether. Antimicrob Agents Chemother. 1985 Apr;27(4):632–639. doi: 10.1128/aac.27.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Hackbarth C. J., Drake T. A., Rusnak M. G., Sande M. A. Endocarditis due to methicillin-resistant Staphylococcus aureus in rabbits: expression of resistance to beta-lactam antibiotics in vivo and in vitro. J Infect Dis. 1984 Jun;149(6):894–903. doi: 10.1093/infdis/149.6.894. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Hackbarth C. J. Effect of NaCl and nafcillin on penicillin-binding protein 2a and heterogeneous expression of methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Dec;31(12):1982–1988. doi: 10.1128/aac.31.12.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles N. W., Gross R. Liberation of surface-located penicillinase from Staphylococcus aureus. Biochem J. 1967 Mar;102(3):742–747. doi: 10.1042/bj1020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto D. W., Johnston J. L., Archer G. L. Molecular epidemiology of trimethoprim resistance among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 Nov;31(11):1683–1688. doi: 10.1128/aac.31.11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Bonner D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):172–175. doi: 10.1128/aac.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX R., SMITH J. T. The nature of penicillin resistance in staphylococci. Lancet. 1961 Sep 2;2(7201):520–522. doi: 10.1016/s0140-6736(61)92958-0. [DOI] [PubMed] [Google Scholar]
- Kim T. K., Chipley J. R. Effect of salts on penicillinase release by Staphylococcus aureus. Microbios. 1974 Jun-Jul;10A SUPPL(41):55–63. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman B. B., Freedman L. R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971 Oct;44(2):206–213. [PMC free article] [PubMed] [Google Scholar]
- Qoronfleh M. W., Wilkinson B. J. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother. 1986 Feb;29(2):250–257. doi: 10.1128/aac.29.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]



