Abstract
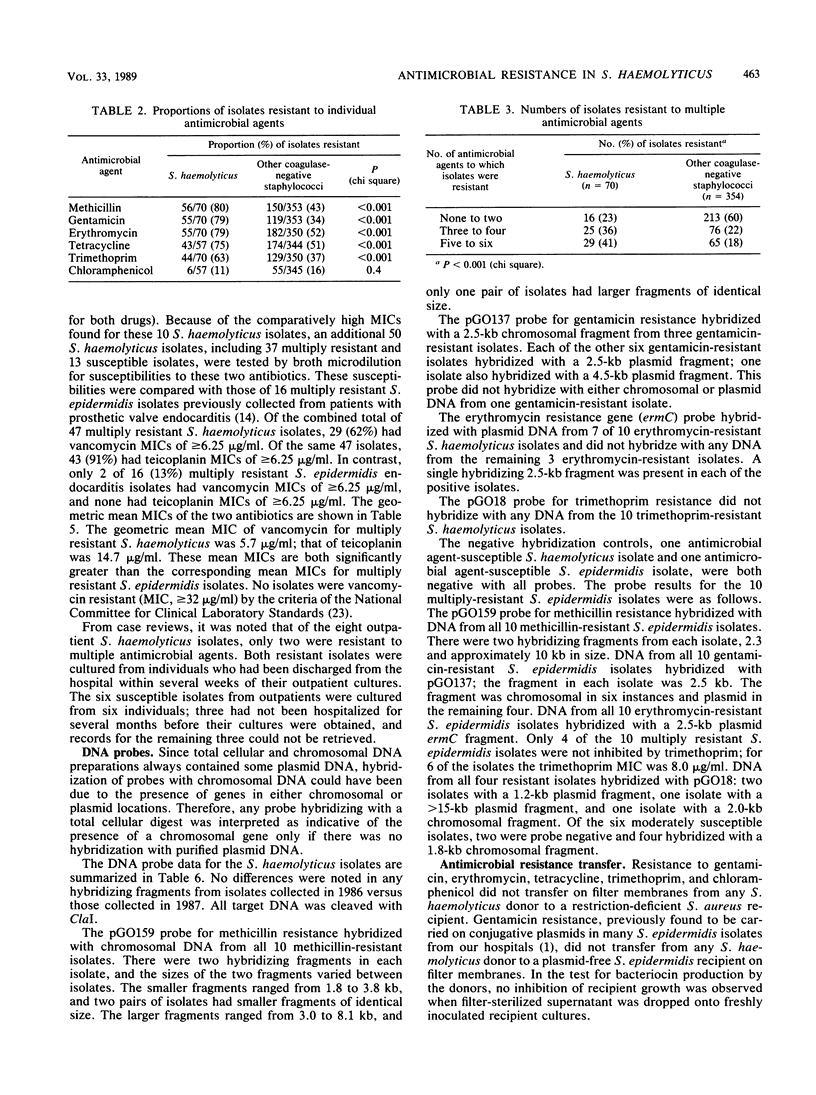
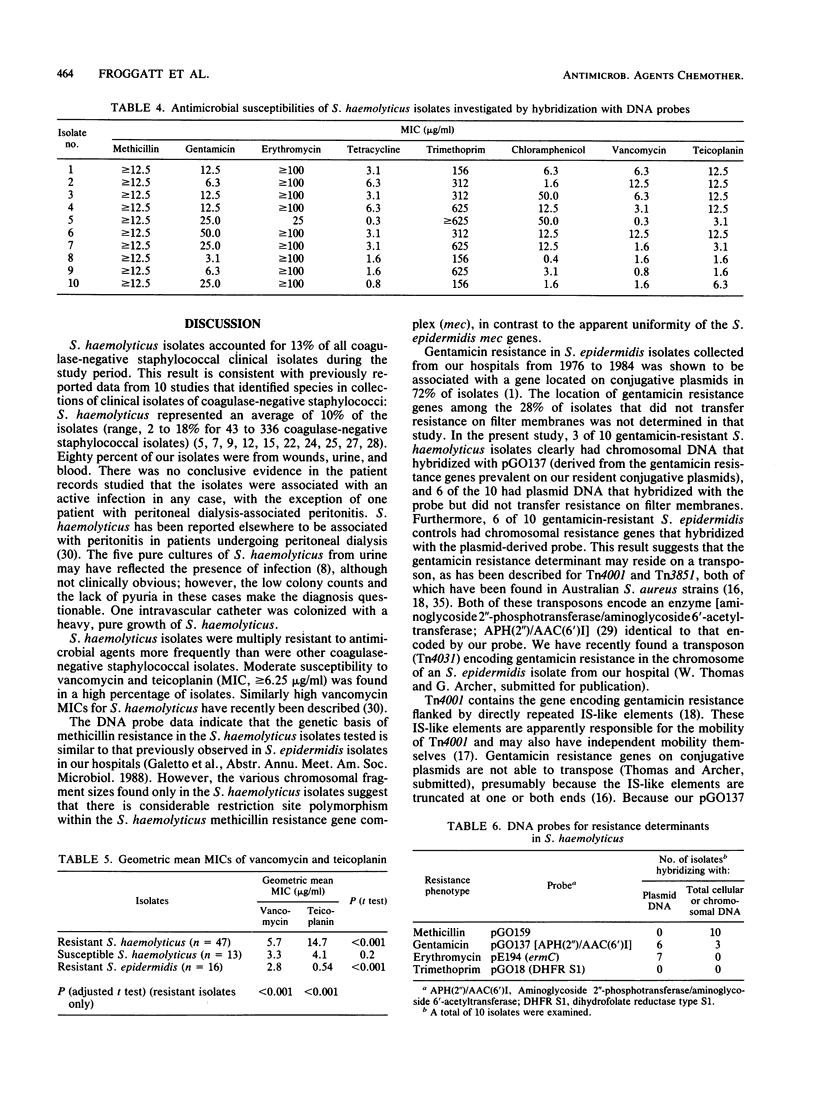
Staphylococcus haemolyticus is frequently cultured from hospitalized patients and is characterized by resistance to multiple antimicrobial agents. We found that S. haemolyticus represented 70 of 524 (13%) coagulase-negative staphylococcal isolates identified by the clinical microbiology laboratories of two hospitals over 2 months. S. haemolyticus isolates were recovered from wounds (44%), urine (26%), blood (10%), and other sources (20%). All S. haemolyticus isolates were tested for susceptibility to six antimicrobial agents; 77% were resistant to three or more agents, and 41% were resistant to five or six agents. In addition, among 47 multiply resistant isolates, high MICs (greater than or equal to 6.25 micrograms/ml) of vancomycin (62% of isolates) and teicoplanin (91% of isolates) were found. DNA probes which were derived from S. epidermidis or S. aureus and which contained sequences associated with resistance to antimicrobial agents were used to detect specific genes in the total cellular and plasmid DNAs of 10 resistant S. haemolyticus isolates. Resistance gene probes and the numbers of resistant isolates hybridizing were as follows: methicillin, 10 of 10; gentamicin, 9 of 10; erythromycin, 7 of 10; and trimethoprim, 0 of 10. Genes for resistance to methicillin were found only in chromosomal locations, genes for resistance to gentamicin were found in both chromosomal and plasmid locations, and genes for resistance to erythromycin were found in plasmid locations only. With the exception of trimethoprim resistance determinants, similar genes were found among concurrently isolated multiply resistant S. epidermidis isolates from our hospitals. S. haemolyticus is a potentially important nosocomial species which readily acquires antimicrobial resistance genes and which shares, to some extent, in a common gene pool with S. epidermidis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Dietrick D. R., Johnston J. L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985 Feb;151(2):243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. D., Berger-Bächi B., Kayser F. H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986 Feb;165(2):373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughter J. P., Johnston J. L., Archer G. L. Characterization of a staphylococcal trimethoprim resistance gene and its product. Antimicrob Agents Chemother. 1987 Jul;31(7):1027–1032. doi: 10.1128/aac.31.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Wang C., Person A., Kiehn T. E., Armstrong D. Species identification of coagulase-negative staphylococcal isolates from blood cultures. J Clin Microbiol. 1982 Mar;15(3):439–442. doi: 10.1128/jcm.15.3.439-442.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto D. W., Johnston J. L., Archer G. L. Molecular epidemiology of trimethoprim resistance among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 Nov;31(11):1683–1688. doi: 10.1128/aac.31.11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruer L. D., Bartlett R., Ayliffe G. A. Species identification and antibiotic sensitivity of coagulase-negative staphylococci from CAPD peritonitis. J Antimicrob Chemother. 1984 Jun;13(6):577–583. doi: 10.1093/jac/13.6.577. [DOI] [PubMed] [Google Scholar]
- Gunn B. A., Davis C. E., Jr Staphylococcus haemolyticus urinary tract infection in a male patient. J Clin Microbiol. 1988 May;26(5):1055–1057. doi: 10.1128/jcm.26.5.1055-1057.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Iliffe A. Antimicrobial resistance in coagulase-negative staphylococci. J Med Microbiol. 1985 Apr;19(2):217–226. doi: 10.1099/00222615-19-2-217. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis B., Matthews P. R., Stewart P. R. The expression in Staphylococcus aureus of cloned DNA encoding methicillin resistance. J Gen Microbiol. 1988 Jun;134(6):1465–1469. doi: 10.1099/00221287-134-6-1465. [DOI] [PubMed] [Google Scholar]
- Iwantscheff A., Kühnen E., Brandis H. Species distribution of coagulase-negative staphylococci isolated from clinical sources. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Aug;260(1):41–50. doi: 10.1016/s0176-6724(85)80096-1. [DOI] [PubMed] [Google Scholar]
- Jenssen W. D., Thakker-Varia S., Dubin D. T., Weinstein M. P. Prevalence of macrolides-lincosamides-streptogramin B resistance and erm gene classes among clinical strains of staphylococci and streptococci. Antimicrob Agents Chemother. 1987 Jun;31(6):883–888. doi: 10.1128/aac.31.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchmer A. W., Archer G. L., Dismukes W. E. Staphylococcus epidermidis causing prosthetic valve endocarditis: microbiologic and clinical observations as guides to therapy. Ann Intern Med. 1983 Apr;98(4):447–455. doi: 10.7326/0003-4819-98-4-447. [DOI] [PubMed] [Google Scholar]
- Leighton P. M., Little J. A. Identification of coagulase-negative Staphylococci isolated from urinary tract infections. Am J Clin Pathol. 1986 Jan;85(1):92–95. doi: 10.1093/ajcp/85.1.92. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Gillespie M. T., Byrne M. E., May J. W., Skurray R. A. Plasmid-mediated resistance to gentamicin in Staphylococcus aureus: the involvement of a transposon. J Med Microbiol. 1987 Mar;23(2):101–110. doi: 10.1099/00222615-23-2-101. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Gillespie M. T., Skurray R. A. Detection and characterization of IS256, an insertion sequence in Staphylococcus aureus. J Gen Microbiol. 1987 Nov;133(11):3031–3038. doi: 10.1099/00221287-133-11-3031. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Weise C. E., Sarafin H. W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977 Jun 9;296(23):1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- Marsik F. J., Brake S. Species identification and susceptibility to 17 antibiotics of coagulase-negative staphylococci isolated from clinical specimens. J Clin Microbiol. 1982 Apr;15(4):640–645. doi: 10.1128/jcm.15.4.640-645.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham C. A., Stempsey W. Incidence, adherence, and antibiotic resistance of coagulase-negative Staphylococcus species causing human disease. Diagn Microbiol Infect Dis. 1984 Sep;2(4):293–299. doi: 10.1016/0732-8893(84)90060-9. [DOI] [PubMed] [Google Scholar]
- Nicolle L. E., Hoban S. A., Harding G. K. Characterization of coagulase-negative staphylococci from urinary tract specimens. J Clin Microbiol. 1983 Feb;17(2):267–271. doi: 10.1128/jcm.17.2.267-271.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi J. T., Robbins J., Lampson B. C., Hecht D. W. Characterization of a macrolide, lincosamide, and streptogramin resistance plasmid in Staphylococcus epidermidis. J Bacteriol. 1981 Nov;148(2):559–564. doi: 10.1128/jb.148.2.559-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett D. A., Welch D. F. Recognition of Staphylococcus saprophyticus in urine cultures by screening colonies for production of phosphatase. J Clin Microbiol. 1985 Mar;21(3):310–313. doi: 10.1128/jcm.21.3.310-313.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon S., Guenthner S. H., Wenzel R. P. Microbiologic studies of coagulase-negative staphylococci isolated from patients with nosocomial bacteraemias. J Hosp Infect. 1986 Mar;7(2):121–129. doi: 10.1016/0195-6701(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tesch W., Strässle A., Berger-Bächi B., O'Hara D., Reynolds P., Kayser F. H. Cloning and expression of methicillin resistance from Staphylococcus epidermidis in Staphylococcus carnosus. Antimicrob Agents Chemother. 1988 Oct;32(10):1494–1499. doi: 10.1128/aac.32.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S., Jenssen W. D., Moon-McDermott L., Weinstein M. P., Dubin D. T. Molecular epidemiology of macrolides-lincosamides-streptogramin B resistance in Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 May;31(5):735–743. doi: 10.1128/aac.31.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Greed L. C., Grubb W. B. Transposition of gentamicin resistance to staphylococcal plasmids encoding resistance to cationic agents. J Antimicrob Chemother. 1984 Aug;14(2):115–124. doi: 10.1093/jac/14.2.115. [DOI] [PubMed] [Google Scholar]


