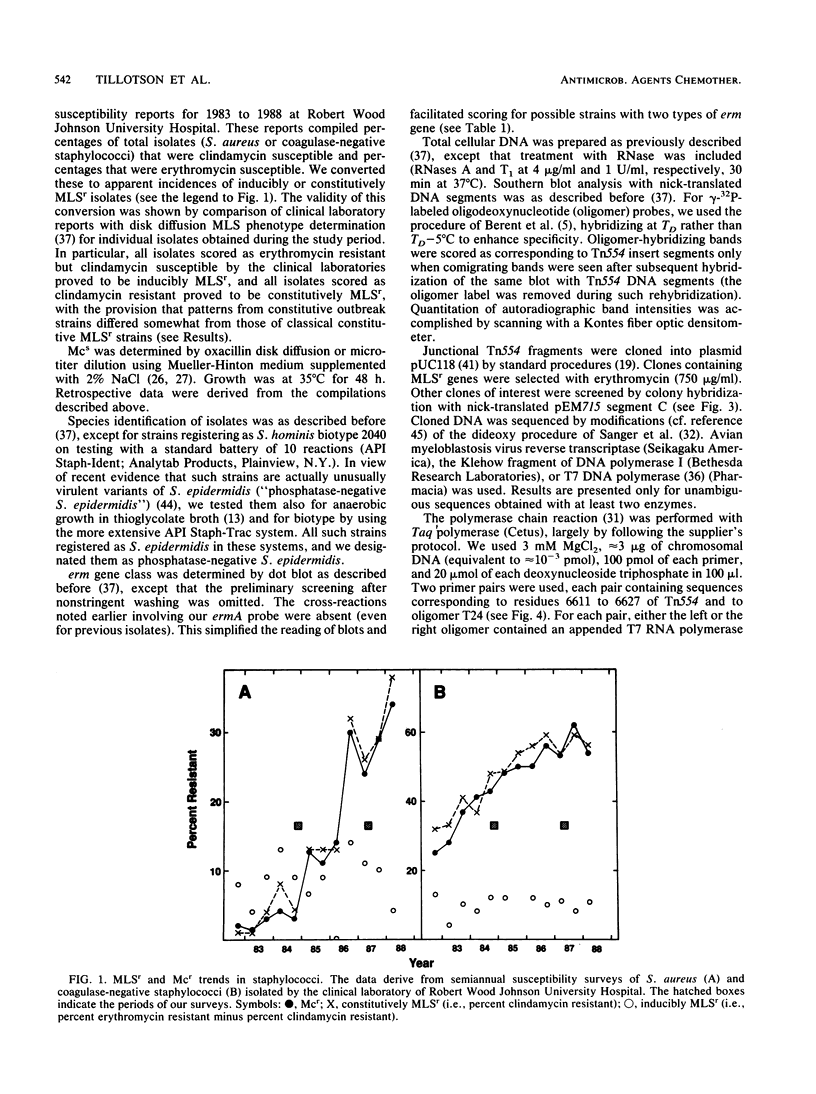
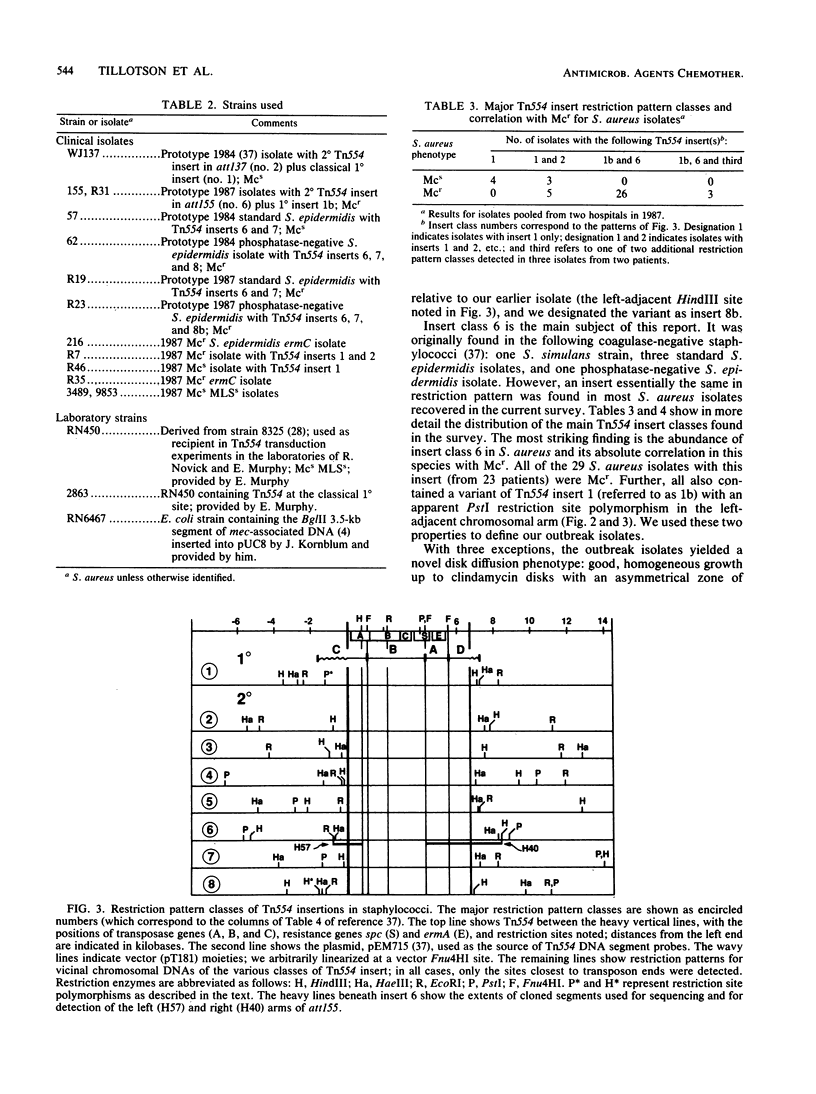
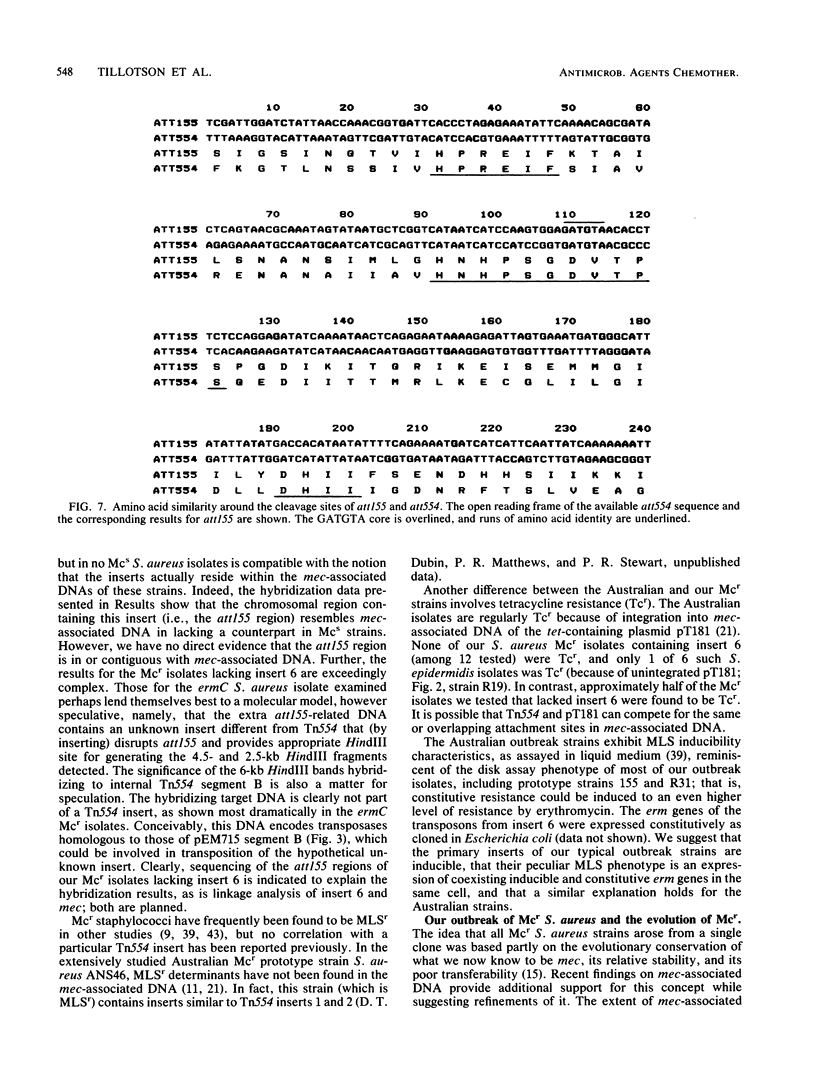
Abstract
Macrolides-lincosamides-streptogramin B resistance in staphylococci can result from a gene, ermA, that comprises part of transposon Tn554. Tn554 is unusual in (i) its high specificity for a primary chromosomal attachment site, att554, and (ii) the variability of its 3'-terminal six or seven nucleotides, which appear to copy the six or seven chromosomal nucleotides 5' to the parent transposon during transposition. We characterized a novel Tn554 insert in the chromosomes of methicillin-resistant Staphylococcus aureus strains involved in a current outbreak. This insert was found to resemble an insert recently discovered in S. epidermidis in its junctional fragment restriction pattern. Sequence analysis of the junctional regions showed that the attachment site, att155, exhibited 78% similarity to att554 (39 of the 50 nucleotides flanking the insertion sites) for both S. aureus and S. epidermidis inserts and that the 3' hexanucleotide of the S. epidermidis transposon (GACATC) resembled the reverse complement (TACATC) of its commonly occurring S. aureus counterpart (GATGTA). Epidemiologic and molecular data indicated that att155 is harbored by extra DNA characteristic of methicillin-resistant strains and absent from methicillin-susceptible ones. Further, Southern hybridization showed that, even in the absence of Tn554 inserts, some methicillin-resistant strains contain DNA related to att155 and Tn554.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L. Molecular epidemiology of multiresistant Staphylococcus epidermidis. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):133–138. doi: 10.1093/jac/21.suppl_c.133. [DOI] [PubMed] [Google Scholar]
- Atkinson B. A., Lorian V. Antimicrobial agent susceptibility patterns of bacteria in hospitals from 1971 to 1982. J Clin Microbiol. 1984 Oct;20(4):791–796. doi: 10.1128/jcm.20.4.791-796.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis-Maino L., Berger-Bächi B., Weber H., Beck W. D., Kayser F. H. IS431, a staphylococcal insertion sequence-like element related to IS26 from Proteus vulgaris. Gene. 1987;59(1):107–113. doi: 10.1016/0378-1119(87)90271-x. [DOI] [PubMed] [Google Scholar]
- Beck W. D., Berger-Bächi B., Kayser F. H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986 Feb;165(2):373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. Antagonisme in vitro entre l'érythromycine et la spiramycine. Ann Inst Pasteur (Paris) 1956 Jun;90(6):787–790. [PubMed] [Google Scholar]
- Chambers H. F. Coagulase-negative staphylococci resistant to beta-lactam antibiotics in vivo produce penicillin-binding protein 2a. Antimicrob Agents Chemother. 1987 Dec;31(12):1919–1924. doi: 10.1128/aac.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmi M., Foresti I., Ravizzola G., Bonfanti C., Verardi R., Caruso A., Turano A. Antibiotic resistances and plasmids in Staphylococcus aureus from Italian hospitals. J Med Microbiol. 1987 Mar;23(2):111–118. doi: 10.1099/00222615-23-2-111. [DOI] [PubMed] [Google Scholar]
- Inglis B., Matthews P. R., Stewart P. R. The expression in Staphylococcus aureus of cloned DNA encoding methicillin resistance. J Gen Microbiol. 1988 Jun;134(6):1465–1469. doi: 10.1099/00221287-134-6-1465. [DOI] [PubMed] [Google Scholar]
- Jenssen W. D., Thakker-Varia S., Dubin D. T., Weinstein M. P. Prevalence of macrolides-lincosamides-streptogramin B resistance and erm gene classes among clinical strains of staphylococci and streptococci. Antimicrob Agents Chemother. 1987 Jun;31(6):883–888. doi: 10.1128/aac.31.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Pattee P. A., Baldwin J. N. Chromosomal map location of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1978 Aug;135(2):460–465. doi: 10.1128/jb.135.2.460-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Kruczenyk S. C. Epidemiology of antibiotic resistance in Staphylococcus aureus. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):207–214. doi: 10.1093/jac/18.supplement_c.207. [DOI] [PubMed] [Google Scholar]
- Lampson B. C., Parisi J. T. Naturally occurring Staphylococcus epidermidis plasmid expressing constitutive macrolide-lincosamide-streptogramin B resistance contains a deleted attenuator. J Bacteriol. 1986 May;166(2):479–483. doi: 10.1128/jb.166.2.479-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Huwyler L., de Freire Bastos M. do C. Transposon Tn554: complete nucleotide sequence and isolation of transposition-defective and antibiotic-sensitive mutants. EMBO J. 1985 Dec 1;4(12):3357–3365. doi: 10.1002/j.1460-2075.1985.tb04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. Inhibition of Tn554 transposition: deletion analysis. Plasmid. 1983 Nov;10(3):260–269. doi: 10.1016/0147-619x(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Murphy E., Löfdahl S. Transposition of Tn554 does not generate a target duplication. Nature. 1984 Jan 19;307(5948):292–294. doi: 10.1038/307292a0. [DOI] [PubMed] [Google Scholar]
- Murphy E., Phillips S., Edelman I., Novick R. P. Tn554: isolation and characterization of plasmid insertions. Plasmid. 1981 May;5(3):292–305. doi: 10.1016/0147-619x(81)90006-8. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Phillips S., Novick R. P. Tn554--a site-specific repressor-controlled transposon in Staphylococcus aureus. Nature. 1979 Mar 29;278(5703):476–478. doi: 10.1038/278476a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner S., Inglis B., Matthews P. R., Stewart P. R. Mercury and tetracycline resistance genes and flanking repeats associated with methicillin resistance on the chromosome of Staphylococcus aureus. Mol Microbiol. 1988 Mar;2(2):289–292. doi: 10.1111/j.1365-2958.1988.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S., Jenssen W. D., Moon-McDermott L., Weinstein M. P., Dubin D. T. Molecular epidemiology of macrolides-lincosamides-streptogramin B resistance in Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 May;31(5):735–743. doi: 10.1128/aac.31.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S., Ranzini A. C., Dubin D. T. Ribosomal RNA methylation in Staphylococcus aureus and Escherichia coli: effect of the "MLS" (erythromycin resistance) methylase. Plasmid. 1985 Sep;14(2):152–161. doi: 10.1016/0147-619x(85)90075-7. [DOI] [PubMed] [Google Scholar]
- Townsend D. E., Grubb W. B., Ashdown N. Genetics of drug resistance in methicillin-resistant Staphylococcus aureus from Australian hospitals. J Hosp Infect. 1983 Dec;4(4):331–337. doi: 10.1016/0195-6701(83)90003-8. [DOI] [PubMed] [Google Scholar]
- Trees D. L., Iandolo J. J. Identification of a Staphylococcus aureus transposon (Tn4291) that carries the methicillin resistance gene(s). J Bacteriol. 1988 Jan;170(1):149–154. doi: 10.1128/jb.170.1.149-154.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression--a review. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]
- Wiedemann B., Kresken M. The incidence and development of resistance in Staphylococcus aureus from three European countries. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):27–34. doi: 10.1093/jac/14.suppl_d.27. [DOI] [PubMed] [Google Scholar]
- Younger J. J., Christensen G. D., Bartley D. L., Simmons J. C., Barrett F. F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987 Oct;156(4):548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]
- el Solh N., Allignet J., Bismuth R., Buret B., Fouace J. M. Conjugative transfer of staphylococcal antibiotic resistance markers in the absence of detectable plasmid DNA. Antimicrob Agents Chemother. 1986 Jul;30(1):161–169. doi: 10.1128/aac.30.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]