Abstract
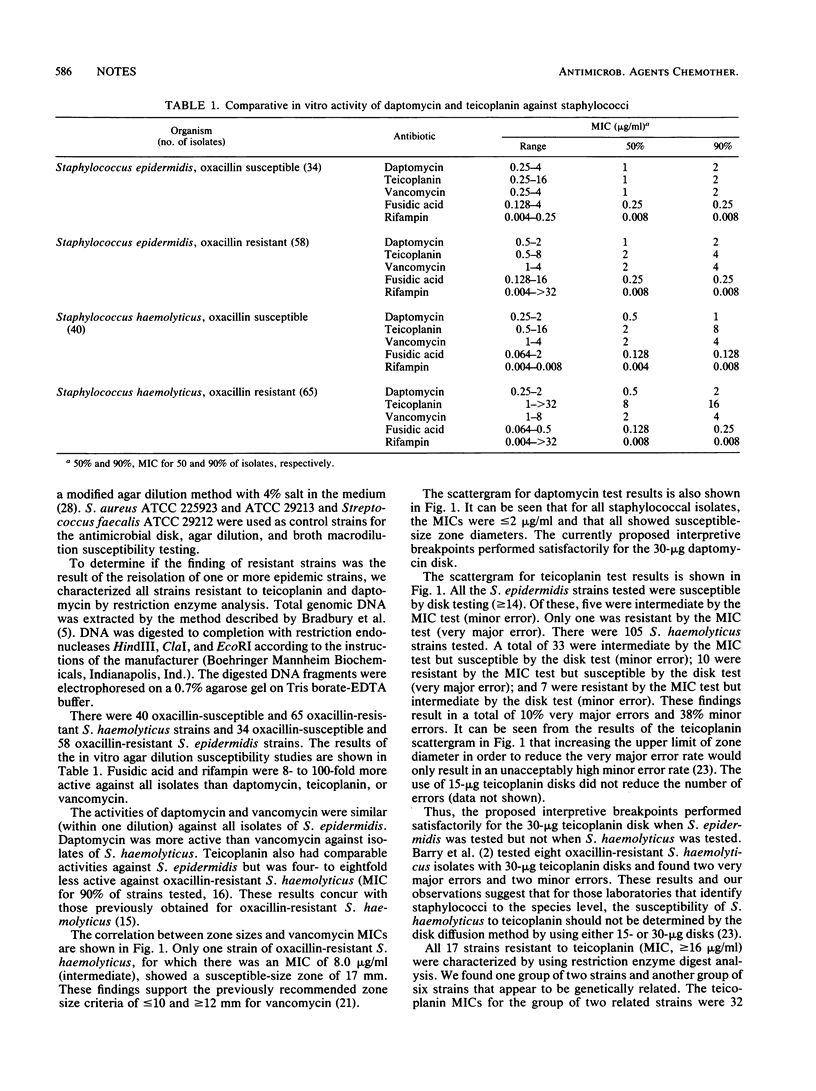
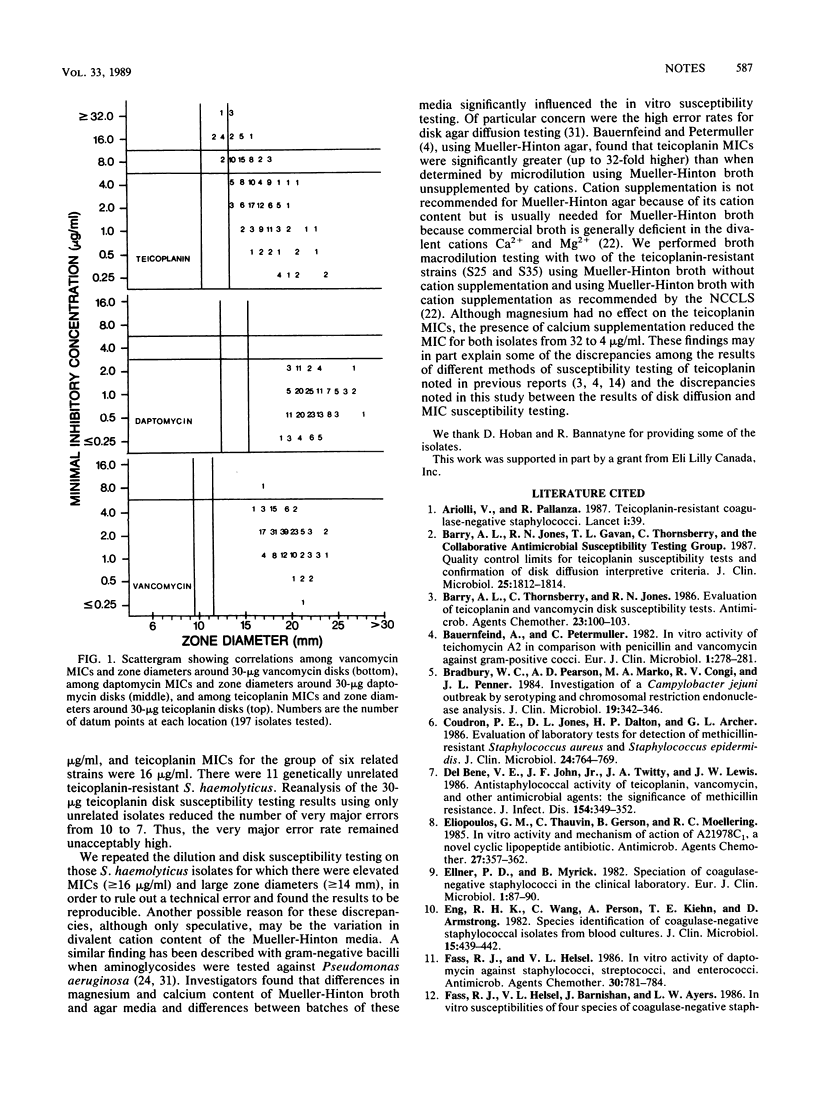
The in vitro activities of daptomycin, teicoplanin, and three other antimicrobial agents were determined against 105 strains of Staphylococcus haemolyticus and 92 strains of Staphylococcus epidermidis. The MICs for 90% of strains tested (MIC90s) of fusidic acid and rifampin were less than or equal to 0.25 microgram/ml. The MIC90s of daptomycin and vancomycin were less than or equal to 4 micrograms/ml. Teicoplanin had a comparable MIC90 of less than or equal to 4 micrograms/ml for isolates of S. epidermidis. However, MIC90s were 8 and 16 micrograms/ml for oxacillin-susceptible and oxacillin-resistant S. haemolyticus, respectively. Disk diffusion tests were evaluated for daptomycin and teicoplanin. Disks with 30 micrograms of teicoplanin performed satisfactorily when S. epidermidis was tested, but when S. haemolyticus was tested, there was a very major error rate of 10% and a minor error rate of 38%.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arioli V., Pallanza R. Teicoplanin-resistant coagulase-negative staphylococci. Lancet. 1987 Jan 3;1(8523):39–39. doi: 10.1016/s0140-6736(87)90724-0. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Gavan T. L., Thornsberry C. Quality control limits for teicoplanin susceptibility tests and confirmation of disk diffusion interpretive criteria. J Clin Microbiol. 1987 Sep;25(9):1812–1814. doi: 10.1128/jcm.25.9.1812-1814.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N. Evaluation of teicoplanin and vancomycin disk susceptibility tests. J Clin Microbiol. 1986 Jan;23(1):100–103. doi: 10.1128/jcm.23.1.100-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind A., Petermüller C. In vitro activity of teichomycin A 2 in comparison with penicillin and vancomycin against gram-positive cocci. Eur J Clin Microbiol. 1982 Oct;1(5):278–281. doi: 10.1007/BF02019971. [DOI] [PubMed] [Google Scholar]
- Bradbury W. C., Pearson A. D., Marko M. A., Congi R. V., Penner J. L. Investigation of a Campylobacter jejuni outbreak by serotyping and chromosomal restriction endonuclease analysis. J Clin Microbiol. 1984 Mar;19(3):342–346. doi: 10.1128/jcm.19.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P. E., Jones D. L., Dalton H. P., Archer G. L. Evaluation of laboratory tests for detection of methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. J Clin Microbiol. 1986 Nov;24(5):764–769. doi: 10.1128/jcm.24.5.764-769.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., John J. F., Jr, Twitty J. A., Lewis J. W. Anti-staphylococcal activity of teicoplanin, vancomycin, and other antimicrobial agents: the significance of methicillin resistance. J Infect Dis. 1986 Aug;154(2):349–352. doi: 10.1093/infdis/154.2.349. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Thauvin C., Gerson B., Moellering R. C., Jr In vitro activity and mechanism of action of A21978C1, a novel cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1985 Mar;27(3):357–362. doi: 10.1128/aac.27.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner P. D., Myrick B. Speciation of coagulase-negative staphylococci in the clinical laboratory. Eur J Clin Microbiol. 1982 Apr;1(2):87–90. doi: 10.1007/BF02014197. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Wang C., Person A., Kiehn T. E., Armstrong D. Species identification of coagulase-negative staphylococcal isolates from blood cultures. J Clin Microbiol. 1982 Mar;15(3):439–442. doi: 10.1128/jcm.15.3.439-442.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L., Barnishan J., Ayers L. W. In vitro susceptibilities of four species of coagulase-negative staphylococci. Antimicrob Agents Chemother. 1986 Oct;30(4):545–552. doi: 10.1128/aac.30.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro activity of LY146032 against staphylococci, streptococci, and enterococci. Antimicrob Agents Chemother. 1986 Nov;30(5):781–784. doi: 10.1128/aac.30.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell C. G., Dawson J. E. Identification of coagulase-negative staphylococci with the API staph system. J Clin Microbiol. 1982 Nov;16(5):874–877. doi: 10.1128/jcm.16.5.874-877.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill V. J., Selepak S. T., Williams E. C. Species identification and antibiotic susceptibilities of coagulase-negative staphylococci isolated from clinical specimens. J Clin Microbiol. 1983 Dec;18(6):1314–1319. doi: 10.1128/jcm.18.6.1314-1319.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. Microbiological properties of teicoplanin. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):1–13. doi: 10.1093/jac/21.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Gruer L. D., Bartlett R., Ayliffe G. A. Species identification and antibiotic sensitivity of coagulase-negative staphylococci from CAPD peritonitis. J Antimicrob Chemother. 1984 Jun;13(6):577–583. doi: 10.1093/jac/13.6.577. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob Agents Chemother. 1987 Apr;31(4):625–629. doi: 10.1128/aac.31.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp C. C., Washington J. A., 2nd Antistaphylococcal activity of a cyclic peptide, LY146032, and vancomycin. Antimicrob Agents Chemother. 1986 Dec;30(6):938–939. doi: 10.1128/aac.30.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsik F. J., Brake S. Species identification and susceptibility to 17 antibiotics of coagulase-negative staphylococci isolated from clinical specimens. J Clin Microbiol. 1982 Apr;15(4):640–645. doi: 10.1128/jcm.15.4.640-645.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Sewell C. M., Clarridge J. E., Young E. J., Guthrie R. K. Clinical significance of coagulase-negative staphylococci. J Clin Microbiol. 1982 Aug;16(2):236–239. doi: 10.1128/jcm.16.2.236-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Henry D. A., Bourgault A. M., Bryan L., Harding G. J., Hoban D. J., Horsman G. B., Marrie T., Turgeon P. Comparison of agar disk diffusion, microdilution broth, and agar dilution for testing antimicrobial susceptibility of coagulase-negative staphylococci. J Clin Microbiol. 1987 Sep;25(9):1741–1746. doi: 10.1128/jcm.25.9.1741-1746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaldo P. E., Debbia E., Schito G. C. In vitro activity of teichomycin and vancomycin alone and in combination with rifampin. Antimicrob Agents Chemother. 1983 Mar;23(3):402–406. doi: 10.1128/aac.23.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. P., O'Hare M. D., Felmingham D., Grüneberg R. N. Teicoplanin-resistant coagulase-negative staphylococcus. Lancet. 1986 Oct 25;2(8513):973–973. doi: 10.1016/s0140-6736(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Woolfrey B. F., Fox J. M., Quall C. O. An analysis of error rates for disc agar-diffusion testing of Pseudomonas aeruginosa versus aminoglycosides. Am J Clin Pathol. 1981 Apr;75(4):559–564. doi: 10.1093/ajcp/75.4.559. [DOI] [PubMed] [Google Scholar]


