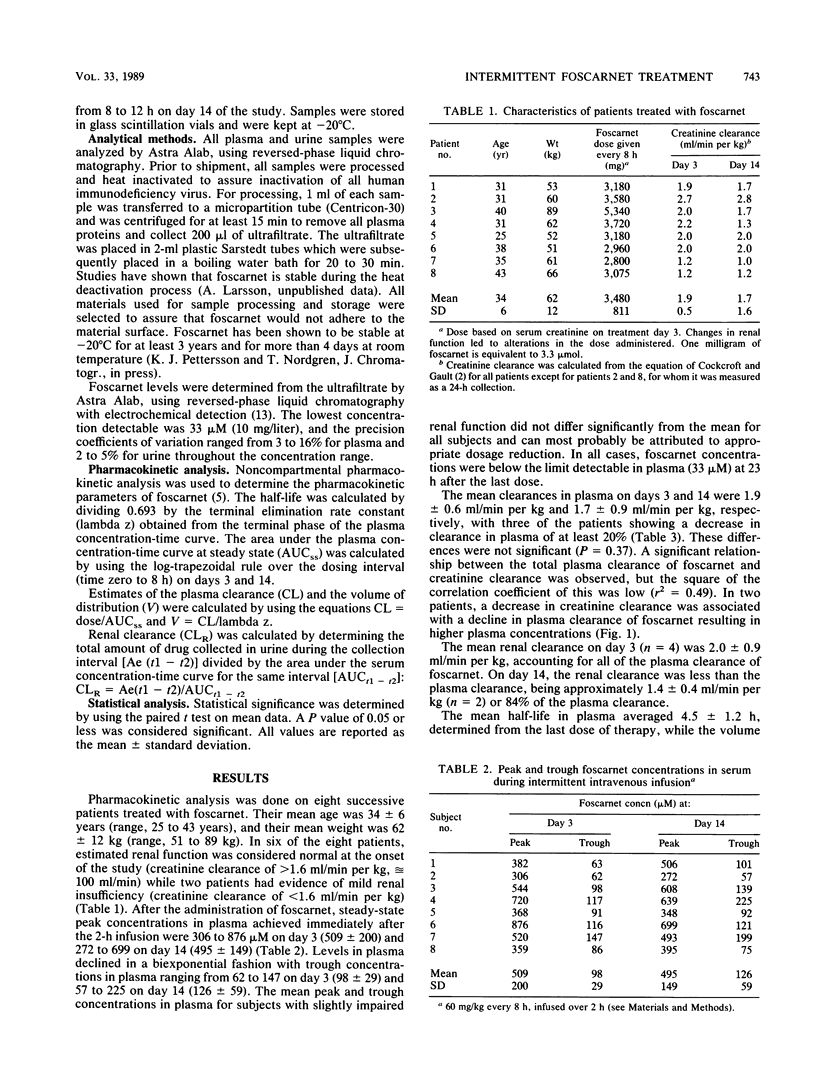
Abstract
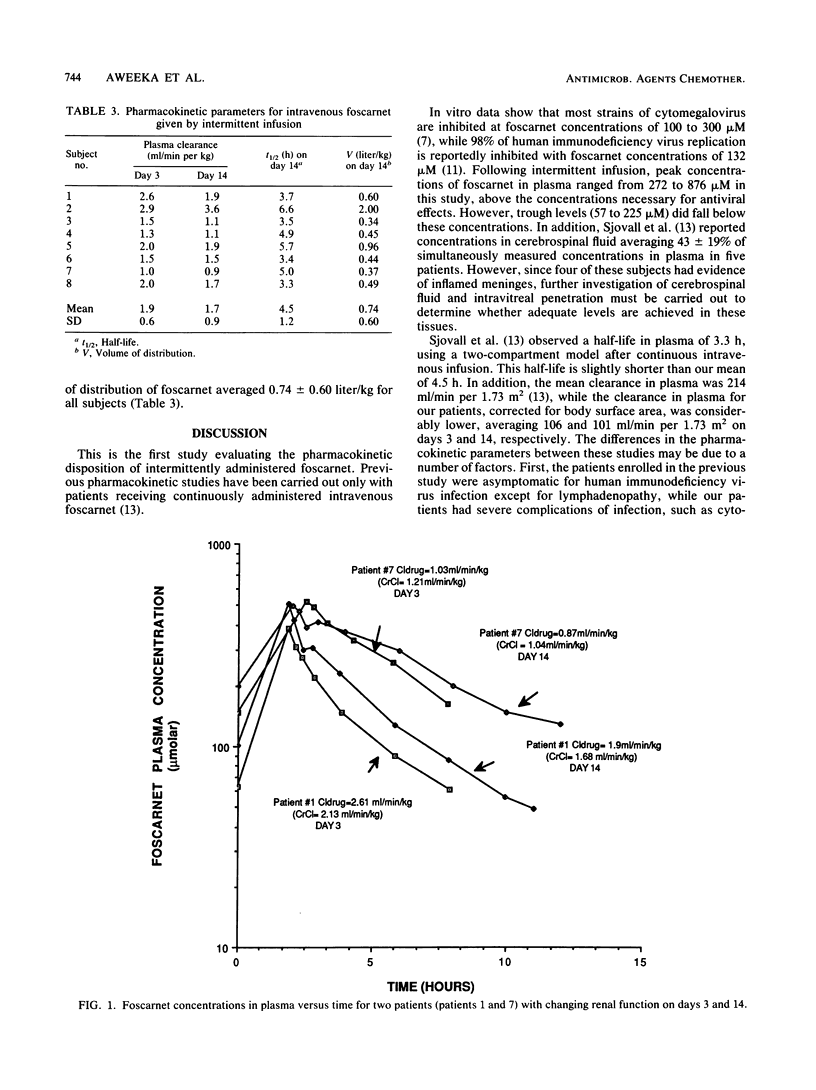
Foscarnet has been shown to be active in vitro against the human immunodeficiency virus and all human herpesviruses including cytomegalovirus (CMV). A pharmacokinetic study was carried out as part of a clinical trial designed to evaluate the safety and efficacy of intermittently administered intravenous foscarnet for the treatment of CMV retinitis. Eight patients with acquired immunodeficiency syndrome and serious CMV retinitis received 2-h intravenous infusions of foscarnet at a dosage of 60 mg/kg of body weight every 8 h for 14 days. Serial plasma samples were collected on days 3 and 14 of therapy, and foscarnet concentrations were determined by high-pressure liquid chromatography. On day 3 of therapy, the mean (+/- standard deviation) peak and trough levels in plasma were 509 (200) and 98 (29) microM, respectively, while on day 14 levels were 495 (149) and 126 (59) microM. The mean clearance in plasma on days 3 and 14 were 1.9 (0.6) and 1.7 (0.9) ml/min per kg, respectively. On day 14, the mean half-life was 4.5 (1.2) h and the volume of distribution was 0.74 (0.60) liter/kg. As the half-life and the clearance of foscarnet in plasma correlated with changes in renal function, dosage adjustments must be made for patients with decreased renal function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cacoub P., Deray G., Baumelou A., Le Hoang P., Rozenbaum W., Gentilini M., Soubrie C., Rousselie R., Jacobs C. Acute renal failure induced by foscarnet: 4 cases. Clin Nephrol. 1988 Jun;29(6):315–318. [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Farthing C., Anderson M. G., Ellis M. E., Gazzard B. G., Chanas A. C. Treatment of cytomegalovirus pneumonitis with foscarnet (trisodium phosphonoformate) in patients with AIDS. J Med Virol. 1987 Jun;22(2):157–162. doi: 10.1002/jmv.1890220206. [DOI] [PubMed] [Google Scholar]
- Gaub J., Pedersen C., Poulsen A. G., Mathiesen L. R., Ulrich K., Lindhardt B. O., Faber V., Gerstoft J., Hofmann B., Lernestedt J. O. The effect of foscarnet (phosphonoformate) on human immunodeficiency virus isolation, T-cell subsets and lymphocyte function in AIDS patients. AIDS. 1987 May;1(1):27–33. [PubMed] [Google Scholar]
- Jacobson M. A., Crowe S., Levy J., Aweeka F., Gambertoglio J., McManus N., Mills J. Effect of Foscarnet therapy on infection with human immunodeficiency virus in patients with AIDS. J Infect Dis. 1988 Oct;158(4):862–865. [PubMed] [Google Scholar]
- Jacobson M. A., O'Donnell J. J., Mills J. Foscarnet treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Antimicrob Agents Chemother. 1989 May;33(5):736–741. doi: 10.1128/aac.33.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintmalm G., Lönnqvist B., Oberg B., Gahrton G., Lernestedt J. O., Lundgren G., Ringdén O., Robert K. H., Wahren B., Groth C. G. Intravenous foscarnet for the treatment of severe cytomegalovirus infection in allograft recipients. Scand J Infect Dis. 1985;17(2):157–163. doi: 10.3109/inf.1985.17.issue-2.06. [DOI] [PubMed] [Google Scholar]
- Oberg B. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol Ther. 1982;19(3):387–415. doi: 10.1016/0163-7258(82)90074-2. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Lönnqvist B., Paulin T., Ahlmén J., Klintmalm G., Wahren B., Lernestedt J. O. Pharmacokinetics, safety and preliminary clinical experiences using foscarnet in the treatment of cytomegalovirus infections in bone marrow and renal transplant recipients. J Antimicrob Chemother. 1986 Mar;17(3):373–387. doi: 10.1093/jac/17.3.373. [DOI] [PubMed] [Google Scholar]
- Sandstrom E. G., Kaplan J. C., Byington R. E., Hirsch M. S. Inhibition of human T-cell lymphotropic virus type III in vitro by phosphonoformate. Lancet. 1985 Jun 29;1(8444):1480–1482. doi: 10.1016/s0140-6736(85)92255-x. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Taguchi Y., Sun D., Thornton A., Gallo R. C., Oberg B. Inhibition of HTLV-III/LAV replication by foscarnet. Biochem Pharmacol. 1985 Nov 15;34(22):4075–4079. doi: 10.1016/0006-2952(85)90392-2. [DOI] [PubMed] [Google Scholar]
- Sjövall J., Karlsson A., Ogenstad S., Sandström E., Saarimäki M. Pharmacokinetics and absorption of foscarnet after intravenous and oral administration to patients with human immunodeficiency virus. Clin Pharmacol Ther. 1988 Jul;44(1):65–73. doi: 10.1038/clpt.1988.114. [DOI] [PubMed] [Google Scholar]
- Walmsley S. L., Chew E., Read S. E., Vellend H., Salit I., Rachlis A., Fanning M. M. Treatment of cytomegalovirus retinitis with trisodium phosphonoformate hexahydrate (Foscarnet). J Infect Dis. 1988 Mar;157(3):569–572. doi: 10.1093/infdis/157.3.569. [DOI] [PubMed] [Google Scholar]


