Abstract
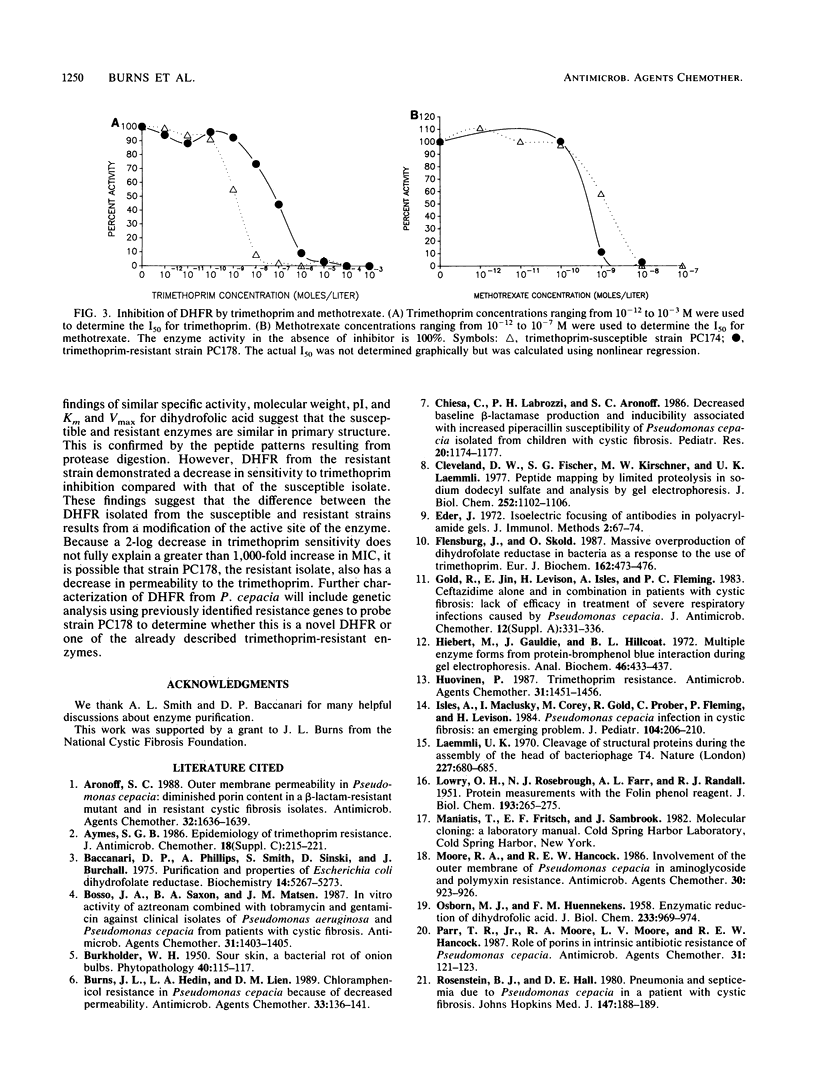
Trimethoprim resistance was investigated in cystic fibrosis isolates of Pseudomonas cepacia. Determination of the MIC of trimethoprim for 111 strains revealed at least two populations of resistant organisms, suggesting the presence of more than one mechanism of resistance. Investigation of the antibiotic target, dihydrofolate reductase, was undertaken in both a susceptible strain and a strain with high-level resistance (MIC, greater than 1,000 micrograms/ml). The enzyme was purified by using ammonium sulfate precipitation, gel filtration, and ion-exchange chromatography. Specific activities, molecular weights, isoelectric points, and substrate kinetics were similar for both enzymes. However, the dihydrofolate reductase from the trimethoprim-resistant strain demonstrated decreased susceptibility to inhibition by trimethoprim and increased susceptibility to inhibition by methotrexate, suggesting that these two enzymes are not identical. We conclude that the mechanism of trimethoprim resistance in this strain with high-level resistance is production of a trimethoprim-resistant dihydrofolate reductase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff S. C. Outer membrane permeability in Pseudomonas cepacia: diminished porin content in a beta-lactam-resistant mutant and in resistant cystic fibrosis isolates. Antimicrob Agents Chemother. 1988 Nov;32(11):1636–1639. doi: 10.1128/aac.32.11.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccanari D., Phillips A., Smith S., Sinski D., Burchall J. Purification and properties of Escherichia coli dihydrofolate reductase. Biochemistry. 1975 Dec 2;14(24):5267–5273. doi: 10.1021/bi00695a006. [DOI] [PubMed] [Google Scholar]
- Bosso J. A., Saxon B. A., Matsen J. M. In vitro activity of aztreonam combined with tobramycin and gentamicin against clinical isolates of Pseudomonas aeruginosa and Pseudomonas cepacia from patients with cystic fibrosis. Antimicrob Agents Chemother. 1987 Sep;31(9):1403–1405. doi: 10.1128/aac.31.9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Hedin L. A., Lien D. M. Chloramphenicol resistance in Pseudomonas cepacia because of decreased permeability. Antimicrob Agents Chemother. 1989 Feb;33(2):136–141. doi: 10.1128/aac.33.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa C., Labrozzi P. H., Aronoff S. C. Decreased baseline beta-lactamase production and inducibility associated with increased piperacillin susceptibility of Pseudomonas cepacia isolated from children with cystic fibrosis. Pediatr Res. 1986 Nov;20(11):1174–1177. doi: 10.1203/00006450-198611000-00026. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Eder J. Isoelectric focusing of antibodies in polyacrylamide gels. J Immunol Methods. 1972 Nov;2(1):67–74. doi: 10.1016/0022-1759(72)90019-1. [DOI] [PubMed] [Google Scholar]
- Flensburg J., Sköld O. Massive overproduction of dihydrofolate reductase in bacteria as a response to the use of trimethoprim. Eur J Biochem. 1987 Feb 2;162(3):473–476. doi: 10.1111/j.1432-1033.1987.tb10664.x. [DOI] [PubMed] [Google Scholar]
- Gold R., Jin E., Levison H., Isles A., Fleming P. C. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):331–336. doi: 10.1093/jac/12.suppl_a.331. [DOI] [PubMed] [Google Scholar]
- Hiebert M., Gauldie J., Hillcoat B. L. Multiple enzyme forms from protein-bromphenol blue interaction during gel electrophoresis. Anal Biochem. 1972 Apr;46(2):433–437. doi: 10.1016/0003-2697(72)90316-8. [DOI] [PubMed] [Google Scholar]
- Huovinen P. Trimethoprim resistance. Antimicrob Agents Chemother. 1987 Oct;31(10):1451–1456. doi: 10.1128/aac.31.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Hancock R. E. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob Agents Chemother. 1986 Dec;30(6):923–926. doi: 10.1128/aac.30.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J., HUENNEKENS F. M. Enzymatic reduction of dihydrofolic acid. J Biol Chem. 1958 Oct;233(4):969–974. [PubMed] [Google Scholar]
- Parr T. R., Jr, Moore R. A., Moore L. V., Hancock R. E. Role of porins in intrinsic antibiotic resistance of Pseudomonas cepacia. Antimicrob Agents Chemother. 1987 Jan;31(1):121–123. doi: 10.1128/aac.31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein B. J., Hall D. E. Pneumonia and septicemia due to Pseudomonas cepacia in a patient with cystic fibrosis. Johns Hopkins Med J. 1980 Nov;147(5):188–189. [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of dihydrofolate reductase genes from trimethoprim-resistant mutants of Escherichia coli. Evidence that dihydrofolate reductase interacts with another essential gene product. Mol Gen Genet. 1982;187(1):72–78. doi: 10.1007/BF00384386. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Klinger J. D., Stern R. C. Pseudomonas cepacia colonization among patients with cystic fibrosis. A new opportunist. Am Rev Respir Dis. 1985 May;131(5):791–796. doi: 10.1164/arrd.1985.131.5.791. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Muszynski M. J., Pai C. H., Marcon M. J., Hribar M. M., Gilligan P. H., Matsen J. M., Ahlin P. A., Hilman B. C., Chartrand S. A. Selective and differential medium for recovery of Pseudomonas cepacia from the respiratory tracts of patients with cystic fibrosis. J Clin Microbiol. 1987 Sep;25(9):1730–1734. doi: 10.1128/jcm.25.9.1730-1734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]