Abstract
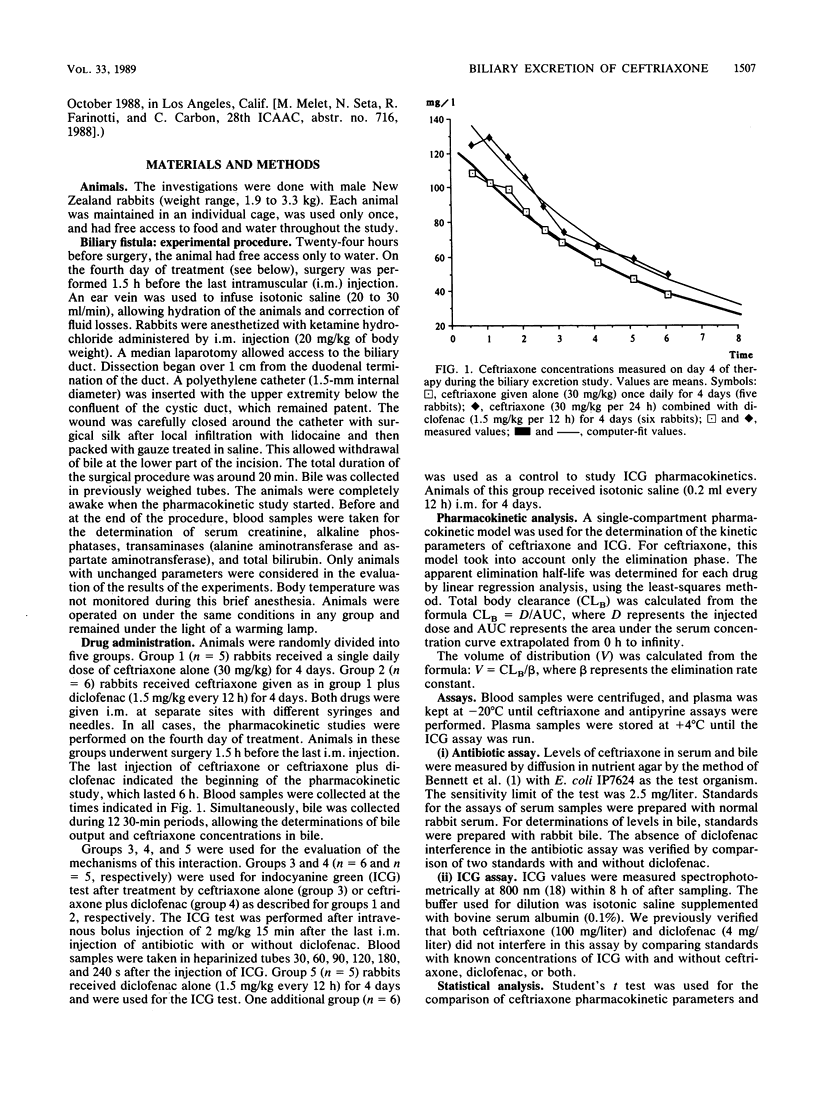
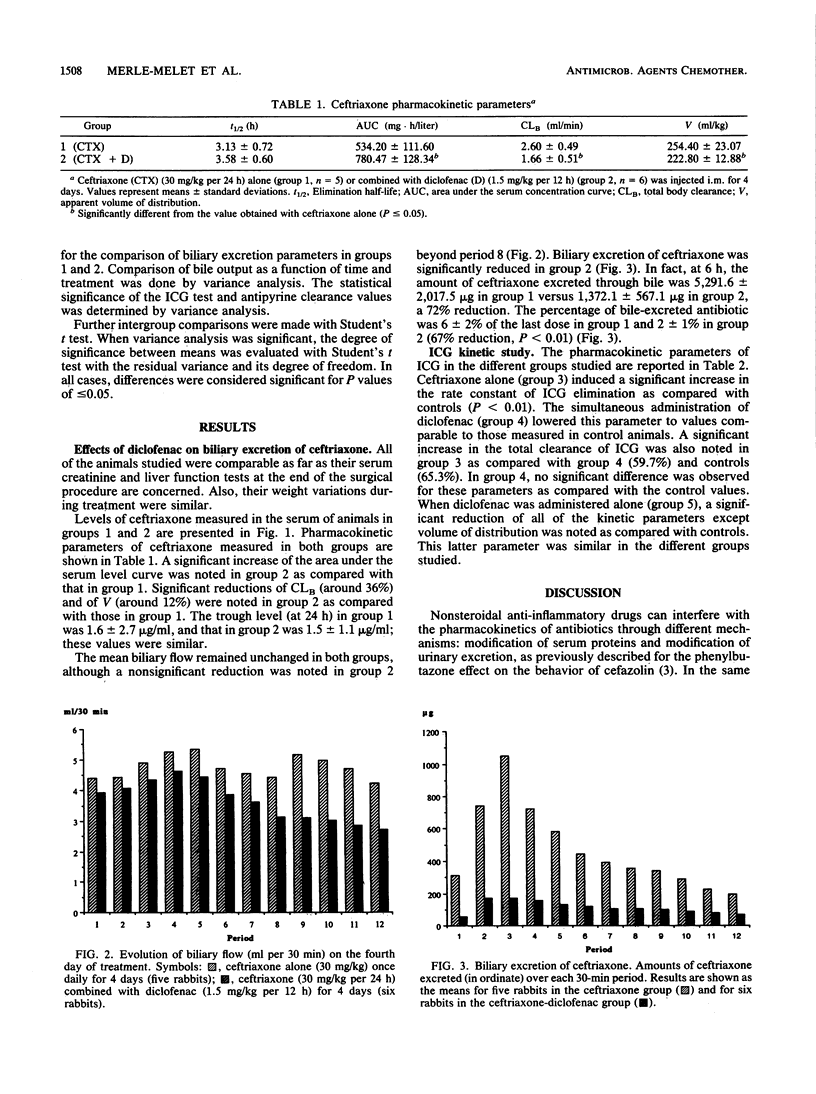
The effects of diclofenac, a nonsteroidal anti-inflammatory drug, on biliary excretion of ceftriaxone were evaluated in rabbits. In a previous study, we demonstrated that diclofenac increased the extravascular diffusion and antibacterial efficacy of ceftriaxone without any effect on serum protein binding and urinary excretion of this antibiotic. We perfected a surgical procedure that allowed the study of biliary secretion in conscious rabbits with a stable hemodynamic state. The kinetic study was carried out on the fourth day of treatment with ceftriaxone alone (30 mg/kg per day given intramuscularly; group 1) or combined with diclofenac (1.5 mg/kg per 12 h given intramuscularly; group 2). Cumulative biliary excretion of ceftriaxone over 6 h was significantly reduced in group 2 (5,291.6 +/- 2,017.5 micrograms in group 1 versus 1,379.1 +/- 567.1 micrograms in group 2). This phenomenon occurred without any change in biliary flow. Indocyanine green clearance (20 mg/kg) increased in animals treated with ceftriaxone alone compared with the saline-treated control group (55.04 +/- 4.68 versus 33.29 +/- 7.52 ml/min per kg, respectively). Diclofenac alone caused a significant decrease in indocyanine green clearance compared with clearance in controls (25.05 +/- 4.74 versus 33.29 +/- 7.52 ml/min per kg), and indocyanine green clearance appeared not significantly different from control values in animals receiving ceftriaxone plus diclofenac. These results suggest that (i) ceftriaxone could increase hepatic blood flow and (ii) reduction of the hepatic clearance of ceftriaxone by diclofenac may be due to hepatic hemodynamic variations involving diclofenac inhibition of prostaglandin synthesis, although an interaction of diclofenac with hepatic uptake of ceftriaxone cannot be ruled out.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogard J. M., Pinget M., Dorner M., Blickle J. F. Biliary elimination of beta-lactam antibiotics by the isolated perfused rabbit liver. Liver. 1985 Jun;5(3):147–155. doi: 10.1111/j.1600-0676.1985.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Carbon C., Contrepois A., Nivoche Y., Grandjean M., Decourt S., Chau N. P. Effects of phenylbutazone on extravascular diffusion, protein binding and urinary excretion of cefazolin in rabbits. J Pharmacol Exp Ther. 1981 Aug;218(2):537–543. [PubMed] [Google Scholar]
- Carbon C., Dromer F., Brion N., Cremieux A. C., Contrepois A. Renal disposition of ceftazidime illustrated by interferences by probenecid, furosemide, and indomethacin in rabbits. Antimicrob Agents Chemother. 1984 Sep;26(3):373–377. doi: 10.1128/aac.26.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D. M., Jefferson G. C. Antipyrine estimations in the rabbit using gas--liquid chromatography: a reliable method for studying factors affecting oxidative drug metabolism. J Pharmacol Methods. 1981 Jan;5(1):59–66. doi: 10.1016/0160-5402(81)90103-0. [DOI] [PubMed] [Google Scholar]
- Feely J., Wood A. J. Effect of inhibitors of prostaglandin synthesis on hepatic drug clearance. Br J Clin Pharmacol. 1983 Jan;15(1):109–111. doi: 10.1111/j.1365-2125.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning C., Holm S. E. Penetration of 14C-Ro 13-9904 into tissue cage fluid in rabbits. Chemotherapy. 1981;27 (Suppl 1):32–36. doi: 10.1159/000238026. [DOI] [PubMed] [Google Scholar]
- Jimenez R., Esteller A., Lopez M. A. Biliary secretion in conscious rabbits: surgical technique. Lab Anim. 1982 Apr;16(2):182–185. doi: 10.1258/002367782781110250. [DOI] [PubMed] [Google Scholar]
- Joly V., Pangon B., Brion N., Vallois J. M., Carbon C. Enhancement of the therapeutic effect of cephalosporins in experimental endocarditis by altering their pharmacokinetics with diclofenac. J Pharmacol Exp Ther. 1988 Aug;246(2):695–700. [PubMed] [Google Scholar]
- Joly V., Pangon B., Vallois J. M., Abel L., Brion N., Bure A., Chau N. P., Contrepois A., Carbon C. Value of antibiotic levels in serum and cardiac vegetations for predicting antibacterial effect of ceftriaxone in experimental Escherichia coli endocarditis. Antimicrob Agents Chemother. 1987 Oct;31(10):1632–1639. doi: 10.1128/aac.31.10.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn A., Eichler H. G., Gasic S. A drug interaction study of ceftriaxone and frusemide in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1986 May;24(5):262–264. [PubMed] [Google Scholar]
- Ohnhaus E. E. Methods of the assessment of the effect of drugs on liver blood flow in man. Br J Clin Pharmacol. 1979 Mar;7(3):223–229. doi: 10.1111/j.1365-2125.1979.tb00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D. M., Heel R. C., Brogden R. N., Speight T. M., Avery G. S. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1984 Jun;27(6):469–527. doi: 10.2165/00003495-198427060-00001. [DOI] [PubMed] [Google Scholar]
- Riess W., Stierlin H., Degen P., Faigle J. W., Gérardin A., Moppert J., Sallmann A., Schmid K., Schweizer A., Sulc M. Pharmacokinetics and metabolism of the anti-inflammatory agent Voltaren. Scand J Rheumatol Suppl. 1978;(22):17–29. doi: 10.3109/03009747809097212. [DOI] [PubMed] [Google Scholar]
- Rissing J. P., Buxton T. B. Effect of ibuprofen on gross pathology, bacterial count, and levels of prostaglandin E2 in experimental staphylococcal osteomyelitis. J Infect Dis. 1986 Oct;154(4):627–630. doi: 10.1093/infdis/154.4.627. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., McLean A. J., duSouich P., Lalka D., Gibaldi M. Nonlinear pharmacokinetics of indocyanine green in the rabbit and rat. J Pharmacokinet Biopharm. 1980 Oct;8(5):483–496. doi: 10.1007/BF01059547. [DOI] [PubMed] [Google Scholar]
- Thiessen J. J., Rappaport P. L., Eppel J. G. Indocyanine green pharmacokinetics in the rabbit. Can J Physiol Pharmacol. 1984 Sep;62(9):1078–1085. doi: 10.1139/y84-180. [DOI] [PubMed] [Google Scholar]
- Tillement J. P., Albengres E., Urien S. Methodological aspects of the evaluation of the binding of drugs to plasma and tissue proteins. Eur J Drug Metab Pharmacokinet. 1979;4(3):123–127. doi: 10.1007/BF03189413. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Rich R., Bray M. A., Zak O., Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987 May;155(5):985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]


