Abstract
Background—The cytokines interleukin 1β (IL-1β) and tumour necrosis factor α (TNF-α) are inhibitors of gastric acid secretion when administered systemically. Aims—To investigate the inhibitory effect of IL-1β and TNF-α on cultured, acid secreting parietal cells in order to determine the mechanism of this inhibition. Methods—Rabbit parietal cells were prepared by collagenase-EDTA digestion and counter flow elutriation. Acid secretory activity was assessed by aminopyrine accumulation. Results—IL-1β and TNF-α inhibited basal and stimulated acid secretion in a dose dependent manner; near maximal effects were seen with both at 10 ng/ml. Inhibition was maximal with 15 minutes pretreatment but seen with up to 18 hours of preincubation. Both cytokines inhibited histamine, carbachol, gastrin, forskolin, and A23187 stimulated acid secretion but had no effect on stimulation by dibutyryl-cAMP. Inhibition of acid secretion was not accompanied by a change in radioligand binding to histamine H2 or gastrin/CCKB receptors. Pertussis toxin abolished the inhibitory effects on histamine and forskolin stimulation. The tyrosine kinase inhibitor herbimycin reduced the inhibitory effects of TNF-α against all stimuli but only reduced the effects of IL-1β against histamine and forskolin stimulation. Conclusions—IL-1β and TNF-α seem to inhibit parietal cell acid secretion by multiple pathways; the inhibition occurs at postreceptor level and involves pertussis toxin and tyrosine kinase dependent and independent pathways. Mucosal production of cytokines may be important in the regulation of gastric acid secretion.
Keywords: acid secretion; aminopyrine; cytokines; interleukin 1β; tumour necrosis factor α; parietal cell
Full Text
The Full Text of this article is available as a PDF (167.5 KB).
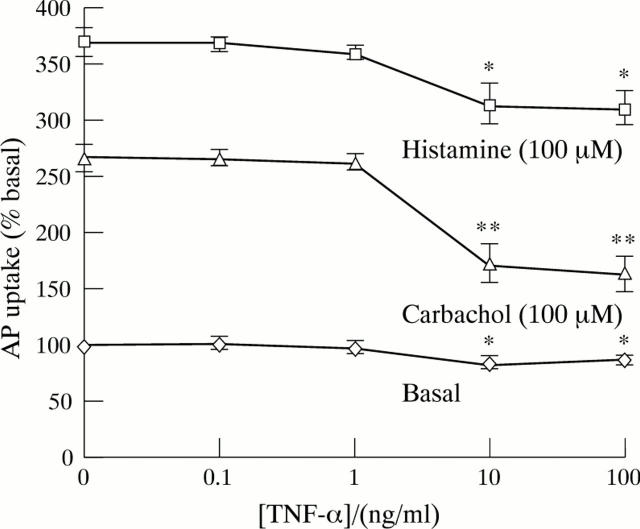
Figure 1 .
Effect of increasing concentrations of TNF-α on aminopyrine (AP) uptake in cultured rabbit parietal cells. Cells were incubated with cytokine for 15 minutes and then aminopyrine accumulation was measured over 30 minutes. Uptake of aminopyrine was measured in the basal state and when stimulated by histamine (10−4 M) and carbachol (10−4 M). Results are expressed as percentage (mean (SEM)) of basal control (n=6). *p<0.05; **p<0.01.
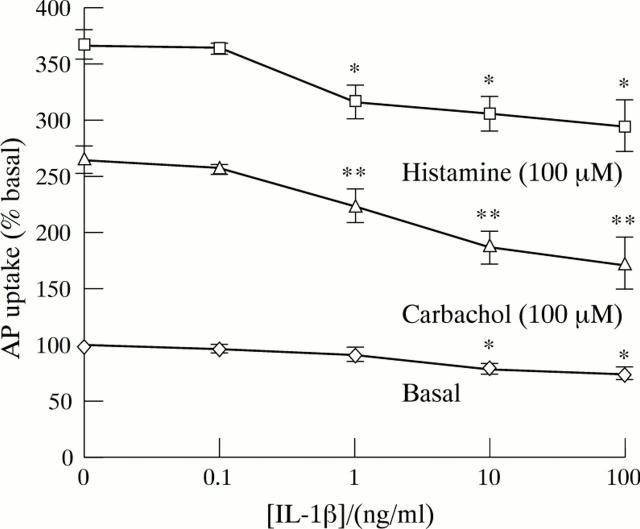
Figure 2 .
Effect of increasing concentrations of IL-1β on aminopyrine (AP) uptake in cultured rabbit parietal cells. Cells were incubated with cytokine for 15 minutes and then aminopyrine accumulation was measured over 30 minutes. Uptake of aminopyrine was measured in the basal state and when stimulated by histamine (10−4 M) and carbachol (10−4 M). Results are expressed as percentage (mean (SEM)) of basal control (n=6). *p<0.05; **p<0.01.
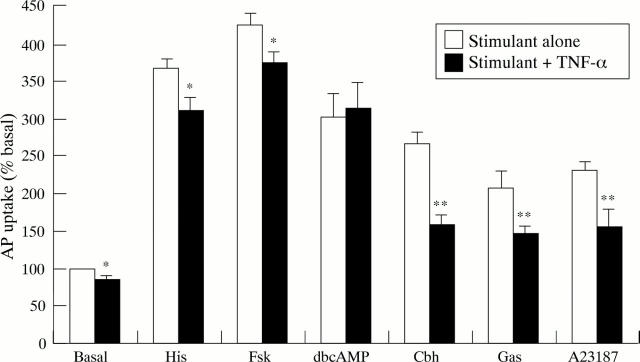
Figure 3 .
Effect of TNF-α (10 ng/ml) on aminopyrine accumulation by parietal cells in the basal state and when stimulated by histamine (His; 10−4 M), forskolin (Fsk; 10−5 M), dibutyryl-cAMP (dbcAMP; 10−4 M), carbachol (Cbh; 10−4 M), gastrin-17 (Gas; 10−7 M), or A23187 (10−6 M). Cells were exposed to TNF-α for 15 minutes before stimulation. Results are expressed as percentage of basal aminopyrine uptake in the absence of cytokine (mean (SEM)), n=5-6. *p<0.05; **p<0.01.
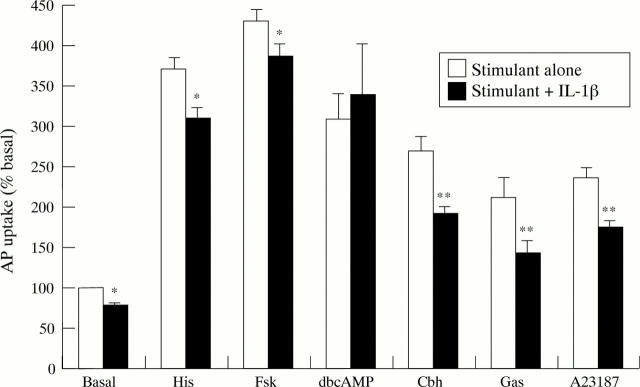
Figure 4 .
Effect of IL-1β (10 ng/ml) on aminopyrine (AP) accumulation by parietal cell in the basal state or stimulated by histamine (His; 10−4 M), forskolin (Fsk; 10−5 M), dibutyryl-cAMP (dbcAMP; 10−4 M), carbachol (Cbh; 10−4 M), gastrin-17 (Gas; 10−7 M), or A23187 (10−6 M). Cells were exposed to IL-1β for 15 minutes before stimulation. Results are expressed as percentage of basal aminopyrine uptake in the absence of cytokine (mean (SEM)), n=5-6. *p<0.05; **p<0.01.
Figure 5 .
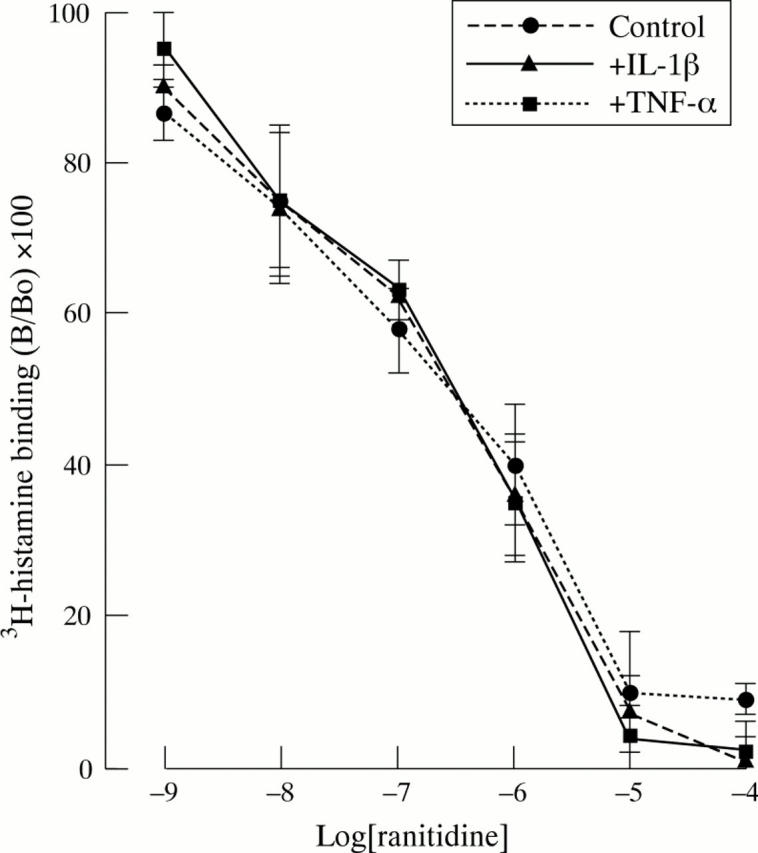
Effect of TNF-α and IL-1β (both 10 ng/ml) on binding of 3H-histamine to rabbit parietal cells. Cells were incubated in appropriate cytokine for 15 minutes before performing the binding experiments. Data are expressed as percentage of binding in the absence of unlabelled histamine (B/Bo). Results are the mean (SEM) of three separate animal preparations.
Figure 6 .
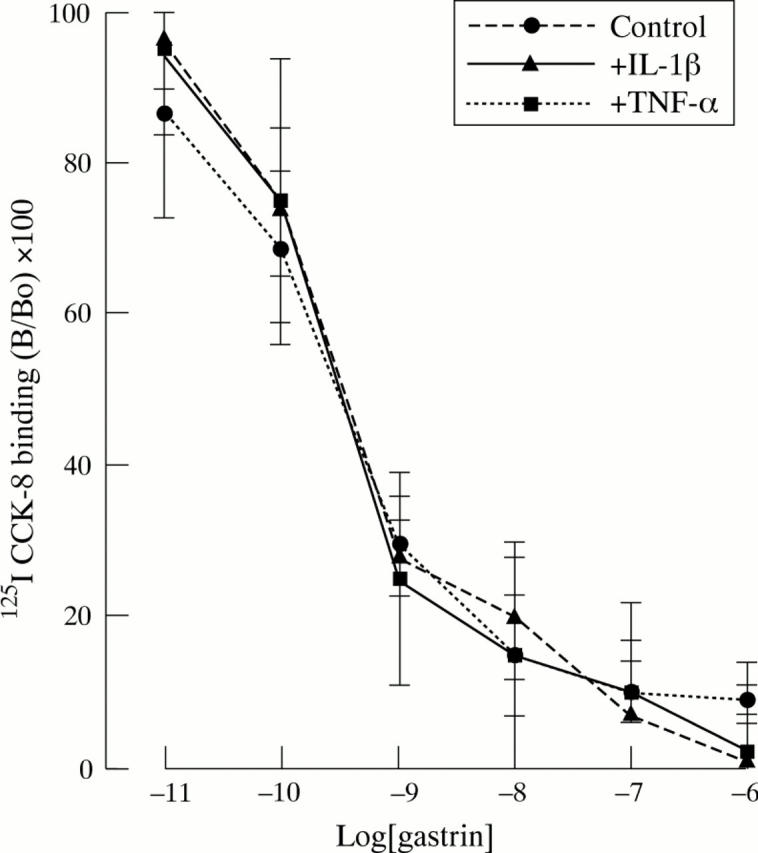
Effect of TNF-α and IL-1β (both 10 ng/ml) on binding of 125I-CCK to rabbit parietal cells. Cells were incubated in appropriate cytokine for 15 minutes before performing the binding experiments. Data are expressed as percentage of binding in the absence of unlabelled gastrin (B/Bo). Results are the mean (SEM) of three separate animal preparations.
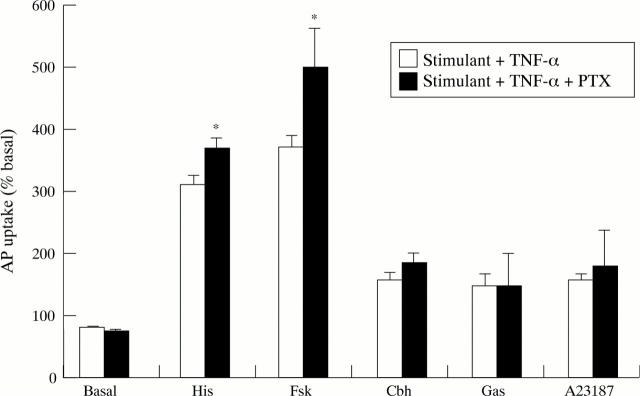
Figure 7 .
Effect of pertussis toxin (PTX; 200 ng/ml) on TNF-α (10 ng/ml) inhibition of aminopyrine (AP) accumulation in the basal state or stimulated by histamine (His; 10−4 M), forskolin (Fsk; 10−5 M), carbachol (Cbh; 10−4 M), gastrin-17 (Gas; 10−7 M), or A23187 (10−6 M). Cells were incubated with PTX for two hours before addition of cytokine. Data are expressed as mean (SEM), n=6. *p<0.05 compared with the effect of TNF-α in the absence of PTX.
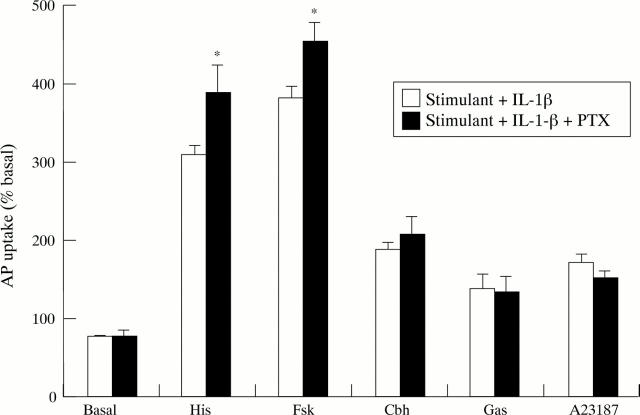
Figure 8 .
Effect of pertussis toxin (PTX; 200 ng/ml) on IL-1β (10 ng/ml) inhibition of aminopyrine (AP) accumulation in the basal state or stimulated by histamine (His; 10−4 M), forskolin (Fsk; 10−5 M), carbachol (Cbh; 10−4 M), gastrin-17 (Gas; 10−7 M), or A23187 (10−6 M). Cells were incubated with PTX for two hours before addition of cytokine. Data are expressed as mean (SEM), n=6. *p<0.05 compared with the effect of IL-1β in the absence of PTX.
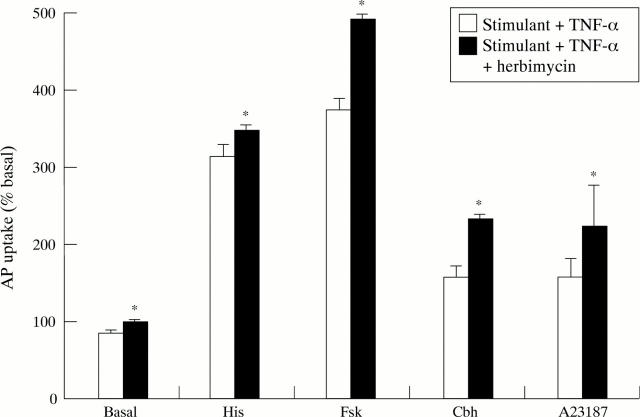
Figure 9 .
Effect of the tyrosine kinase inhibitor herbimycin (10−6 M) on TNF-α (10 ng/ml) induced inhibition of aminopyrine (AP) uptake in rabbit parietal cells in the basal state or stimulated with histamine (His; 10−4 M), forskolin (Fsk; 10−5 M), carbachol (Cbh; 10−4 M), or A23187 (10−6 M). Herbimycin was added 60 minutes before TNF-α. Data are expressed as mean (SEM), n=4. *p<0.05 compared with the effect of TNF-α in the absence of herbimycin.
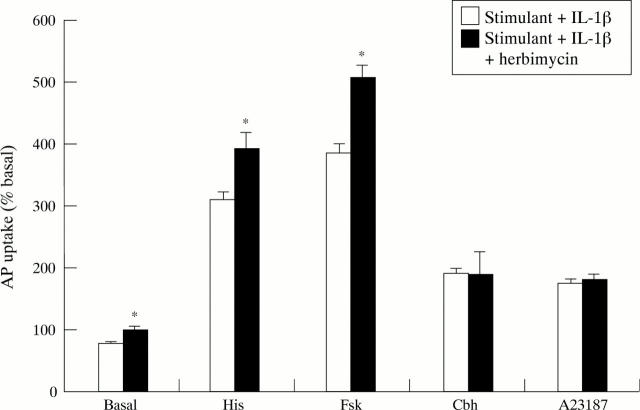
Figure 10 .
Effect of herbimycin (10−6 M) on IL-1β (10 µg/ml) inhibition of aminopyrine (AP) accumulation in rabbit parietal cells in the basal state or stimulated with histamine (His; 10−4 M), forskolin (Fsk; 10−5 M), carbachol (Cbh; 10−4 M), or A23187 (10−6 M). Herbimycin was added 60 minutes before IL-1β. Data are expressed as mean (SEM), n=5. *p<0.05 compared with effect of IL-1β in the absence of herbimycin.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berglindh T., Helander H. F., Obrink K. J. Effects of secretagogues on oxygen consumption, aminopyrine accumulation and morphology in isolated gastric glands. Acta Physiol Scand. 1976 Aug;97(4):401–414. doi: 10.1111/j.1748-1716.1976.tb10281.x. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992 Feb;102(2):720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- Calam J. Helicobacter pylori. Eur J Clin Invest. 1994 Aug;24(8):501–510. doi: 10.1111/j.1365-2362.1994.tb01099.x. [DOI] [PubMed] [Google Scholar]
- Campbell V. W., Del Valle J., Hawn M., Park J., Yamada T. Carbonic anhydrase II gene expression in isolated canine gastric parietal cells. Am J Physiol. 1989 Mar;256(3 Pt 1):G631–G636. doi: 10.1152/ajpgi.1989.256.3.G631. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Hersey S. J., Sachs G., Berglindh T. Histamine responsiveness of isolated gastric glands. Am J Physiol. 1980 Apr;238(4):G312–G320. doi: 10.1152/ajpgi.1980.238.4.G312. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Ljungström M., Smolka A., Brown M. R. Primary culture of secretagogue-responsive parietal cells from rabbit gastric mucosa. Am J Physiol. 1989 Jan;256(1 Pt 1):G254–G263. doi: 10.1152/ajpgi.1989.256.1.G254. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Nakamura K., Petropoulos A. C. Multiple actions of epidermal growth factor and TGF-alpha on rabbit gastric parietal cell function. Am J Physiol. 1994 Nov;267(5 Pt 1):G818–G826. doi: 10.1152/ajpgi.1994.267.5.G818. [DOI] [PubMed] [Google Scholar]
- Chiba T., Fisher S. K., Agranoff B. W., Yamada T. Autoregulation of muscarinic and gastrin receptors on gastric parietal cells. Am J Physiol. 1989 Feb;256(2 Pt 1):G356–G363. doi: 10.1152/ajpgi.1989.256.2.G356. [DOI] [PubMed] [Google Scholar]
- Chiba T., Fisher S. K., Park J., Seguin E. B., Agranoff B. W., Yamada T. Carbamoylcholine and gastrin induce inositol lipid turnover in canine gastric parietal cells. Am J Physiol. 1988 Jul;255(1 Pt 1):G99–105. doi: 10.1152/ajpgi.1988.255.1.G99. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I., Schäffer M., DelValle J., Logsdon C., Campbell V., Uhler M., Yamada T. Molecular cloning of a gene encoding the histamine H2 receptor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):429–433. doi: 10.1073/pnas.88.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Alpert L. C., Smith J. L., Yoshimura H. H. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am J Gastroenterol. 1988 Sep;83(9):974–980. [PubMed] [Google Scholar]
- Katz M. S., Gutierrez G. E., Mundy G. R., Hymer T. K., Caulfield M. P., McKee R. L. Tumor necrosis factor and interleukin 1 inhibit parathyroid hormone-responsive adenylate cyclase in clonal osteoblast-like cells by down-regulating parathyroid hormone receptors. J Cell Physiol. 1992 Oct;153(1):206–213. doi: 10.1002/jcp.1041530125. [DOI] [PubMed] [Google Scholar]
- Kondo S., Shinomura Y., Kanayama S., Kawabata S., Miyazaki Y., Imamura I., Fukui H., Matsuzawa Y. Interleukin-1 beta inhibits gastric histamine secretion and synthesis in the rat. Am J Physiol. 1994 Dec;267(6 Pt 1):G966–G971. doi: 10.1152/ajpgi.1994.267.6.G966. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Louw J. A., Falck V., van Rensburg C., Zak J., Adams G., Marks I. N. Distribution of Helicobacter pylori colonisation and associated gastric inflammatory changes: difference between patients with duodenal and gastric ulcers. J Clin Pathol. 1993 Aug;46(8):754–756. doi: 10.1136/jcp.46.8.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J., Armstrong J. A., McGechie D. B., Glancy R. J. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985 Apr 15;142(8):436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- Morris A., Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987 Mar;82(3):192–199. [PubMed] [Google Scholar]
- Nakatsuji K., Kii Y., Fujitani B., Ito T. General pharmacology of recombinant human tumor necrosis factor. 1st communication: effects on cardiovascular, gastrointestinal, renal and blood functions. Arzneimittelforschung. 1990 Feb;40(2 Pt 1):218–225. [PubMed] [Google Scholar]
- Noach L. A., Bosma N. B., Jansen J., Hoek F. J., van Deventer S. J., Tytgat G. N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994 May;29(5):425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- Nompleggi D. J., Beinborn M., Roy A., Wolfe M. M. The effect of recombinant cytokines on [14C]-aminopyrine accumulation by isolated canine parietal cells. J Pharmacol Exp Ther. 1994 Aug;270(2):440–445. [PubMed] [Google Scholar]
- Park J., Chiba T., Yamada T. Mechanisms for direct inhibition of canine gastric parietal cells by somatostatin. J Biol Chem. 1987 Oct 15;262(29):14190–14196. [PubMed] [Google Scholar]
- Ramsey E. J., Carey K. V., Peterson W. L., Jackson J. J., Murphy F. K., Read N. W., Taylor K. B., Trier J. S., Fordtran J. S. Epidemic gastritis with hypochlorhydria. Gastroenterology. 1979 Jun;76(6):1449–1457. [PubMed] [Google Scholar]
- Robert A., Olafsson A. S., Lancaster C., Zhang W. R. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, and retards gastric emptying. Life Sci. 1991;48(2):123–134. doi: 10.1016/0024-3205(91)90405-z. [DOI] [PubMed] [Google Scholar]
- Saperas E. S., Yang H., Rivier C., Taché Y. Central action of recombinant interleukin-1 to inhibit acid secretion in rats. Gastroenterology. 1990 Dec;99(6):1599–1606. doi: 10.1016/0016-5085(90)90463-b. [DOI] [PubMed] [Google Scholar]
- Schuerer-Maly C. C., Emmenegger U., Ott K., Flogerzi B., Halter F. Interleukin-1 and experimental gastric ulcer healing in the rat. J Physiol Pharmacol. 1993 Mar;44(1):23–29. [PubMed] [Google Scholar]
- Shibasaki T., Yamauchi N., Hotta M., Imaki T., Oda T., Ling N., Demura H. Interleukin-1 inhibits stress-induced gastric erosion in rats. Life Sci. 1991;48(23):2267–2273. doi: 10.1016/0024-3205(91)90342-9. [DOI] [PubMed] [Google Scholar]
- Sobala G. M., Crabtree J. E., Dixon M. F., Schorah C. J., Taylor J. D., Rathbone B. J., Heatley R. V., Axon A. T. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991 Nov;32(11):1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I., Bado A., Moizo L., Laigneau J. P., Tarrade T., Braquet P., Lewin M. J. Platelet-activating factor stimulates gastric acid secretion in isolated rabbit gastric glands. Am J Physiol. 1995 Jun;268(6 Pt 1):G889–G894. doi: 10.1152/ajpgi.1995.268.6.G889. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Amirian D. A., Thomas L. P., Reedy T. J., Elashoff J. D. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. Secretagogue stimulation of [14C]aminopyrine accumulation by isolated canine parietal cells. Am J Physiol. 1980 Apr;238(4):G366–G375. doi: 10.1152/ajpgi.1980.238.4.G366. [DOI] [PubMed] [Google Scholar]
- Soll A. H. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):370–380. doi: 10.1172/JCI108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A. M. G proteins in clinical medicine. Hosp Pract (Off Ed) 1988 Jun 15;23(6):93-100, 105-6, 111-2. doi: 10.1080/21548331.1988.11703488. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Goeddel D. V., Reynolds C., Figari I. S., Weber R. F., Fendly B. M., Palladino M. A., Jr Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol. 1993 Nov 1;151(9):4637–4641. [PubMed] [Google Scholar]
- Vilcek J., Lee T. H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991 Apr 25;266(12):7313–7316. [PubMed] [Google Scholar]
- Wallace J. L., Cucala M., Mugridge K., Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991 Oct;261(4 Pt 1):G559–G564. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Cucala M., Mugridge K. G., Parente L. Mechanisms underlying the protective effects of interleukin 1 in experimental nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 1992 Apr;102(4 Pt 1):1176–1185. [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Mugridge K. G., Parente L. Reduction of the severity of experimental gastric and duodenal ulceration by interleukin-1 beta. Eur J Pharmacol. 1990 Sep 21;186(2-3):279–284. doi: 10.1016/0014-2999(90)90444-b. [DOI] [PubMed] [Google Scholar]
- Wang L., Lucey M. R., Fras A. M., Wilson E. J., Del Valle J. Epidermal growth factor and transforming growth factor-alpha directly inhibit parietal cell function through a similar mechanism. J Pharmacol Exp Ther. 1993 Apr;265(1):308–313. [PubMed] [Google Scholar]
- Wang L., Wilson E. J., Osburn J., DelValle J. Epidermal growth factor inhibits carbachol-stimulated canine parietal cell function via protein kinase C. Gastroenterology. 1996 Feb;110(2):469–477. doi: 10.1053/gast.1996.v110.pm8566594. [DOI] [PubMed] [Google Scholar]
- Weber C., Negrescu E., Erl W., Pietsch A., Frankenberger M., Ziegler-Heitbrock H. W., Siess W., Weber P. C. Inhibitors of protein tyrosine kinase suppress TNF-stimulated induction of endothelial cell adhesion molecules. J Immunol. 1995 Jul 1;155(1):445–451. [PubMed] [Google Scholar]
- el-Omar E. M., Penman I. D., Ardill J. E., Chittajallu R. S., Howie C., McColl K. E. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995 Sep;109(3):681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]