Abstract
Background—VacA and CagA proteins have been reported to be major virulence factors of Helicobacter pylori. However, antibodies against these proteins are frequently found in the sera of Japanese patients regardless of their gastroduodenal status. Aim—To evaluate the expression of VacA and CagA proteins by H pylori strains isolated in Japan. Methods—By using specific antibodies raised against recombinant VacA and CagA proteins, the expression of VacA and CagA was evaluated in 68 H pylori strains isolated from Japanese patients; a vacuolating assay and genotyping of the vacA gene were also used in the evaluation. The results were analysed in relation to the gastroduodenal diseases of the hosts. Results—VacA and CagA proteins were expressed in 59/68 (87%) and in 61/68 (90%) isolates respectively. The vacuolating assay was positive in 57/68 (84%) isolates, indicating that most immunologically VacA positive strains produced active cytotoxin. The prevalence of infection with strains expressing CagA and positive for vacuolating activity (Type I) was very high, 54/68 (79%), irrespective of the gastroduodenal status of the host. Conclusion—Most H pylori isolates in Japan are positive for vacuolating cytotoxin and CagA, and thus these virulence factors cannot be used as markers to discern the risk of developing serious gastroduodenal pathologies in the hosts. However, the high prevalence of infection with strains positive for vacuolating cytotoxin and CagA may contribute to the characteristics of H pylori infection in Japan.
Keywords: VacA; Helicobacter pylori; CagA; ulcer; non-ulcer dyspepsia
Full Text
The Full Text of this article is available as a PDF (116.7 KB).
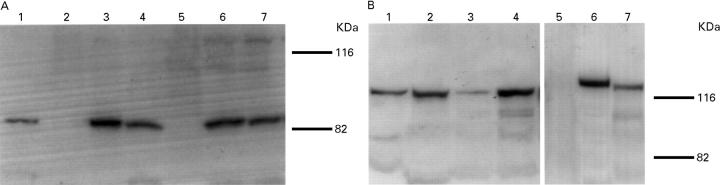
Figure 1 .
(A) Immunoblot analysis of VacA. Surface proteins obtained from seven H pylori strains were probed with antiserum against the recombinant VacA protein. VacA protein was expressed in T-1 (lane 1), T-5, T-6 (lanes 3 and 4), T-24 and T-39 (lanes 6 and 7), but not in T-48 (lane 2) or T-25 (lane 5). (B) Immunoblot analysis of CagA. Total lysates obtained from seven H pylori strains were probed with antiserum against the recombinant CagA protein. CagA protein was expressed in T-1, T-48, T-5, T-6 (lanes 1-4), T-24 and T-39 (lanes 6 and 7), but not in T-25 (lane 5). Molecular mass is shown in kDa.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asaka M., Kimura T., Kato M., Kudo M., Miki K., Ogoshi K., Kato T., Tatsuta M., Graham D. Y. Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer. 1994 Jun 1;73(11):2691–2694. doi: 10.1002/1097-0142(19940601)73:11<2691::aid-cncr2820731107>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Asaka M., Kimura T., Kudo M., Takeda H., Mitani S., Miyazaki T., Miki K., Graham D. Y. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992 Mar;102(3):760–766. doi: 10.1016/0016-5085(92)90156-s. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Cao P., Peek R. M., Jr, Tummuru M. K., Blaser M. J., Cover T. L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995 Jul 28;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995 May 15;55(10):2111–2115. [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C. K., Wong B. C., Kwok E., Ong L., Covacci A., Lam S. K. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996 May;91(5):949–953. [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992 May 25;267(15):10570–10575. [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Glupczynski Y., Lage A. P., Burette A., Tummuru M. K., Perez-Perez G. I., Blaser M. J. Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol. 1995 Jun;33(6):1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Figura N., Taylor J. D., Bugnoli M., Armellini D., Tompkins D. S. Expression of 120 kilodalton protein and cytotoxicity in Helicobacter pylori. J Clin Pathol. 1992 Aug;45(8):733–734. doi: 10.1136/jcp.45.8.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Correa P., Taylor N. S., Thompson N., Fontham E., Janney F., Sobhan M., Ruiz B., Hunter F. High prevalence and persistence of cytotoxin-positive Helicobacter pylori strains in a population with high prevalence of atrophic gastritis. Am J Gastroenterol. 1992 Nov;87(11):1554–1560. [PubMed] [Google Scholar]
- Graham D. Y., Lew G. M., Klein P. D., Evans D. G., Evans D. J., Jr, Saeed Z. A., Malaty H. M. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992 May 1;116(9):705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- Ito Y., Azuma T., Ito S., Miyaji H., Hirai M., Yamazaki Y., Sato F., Kato T., Kohli Y., Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997 Jul;35(7):1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leunk R. D. Production of a cytotoxin by Helicobacter pylori. Rev Infect Dis. 1991 Jul-Aug;13 (Suppl 8):S686–S689. doi: 10.1093/clinids/13.supplement_8.s686. [DOI] [PubMed] [Google Scholar]
- Maeda S., Kanai F., Ogura K., Yoshida H., Ikenoue T., Takahashi M., Kawabe T., Shiratori Y., Omata M. High seropositivity of anti-CagA antibody in Helicobacter pylori-infected patients irrelevant to peptic ulcers and normal mucosa in Japan. Dig Dis Sci. 1997 Sep;42(9):1841–1847. doi: 10.1023/a:1018846723379. [DOI] [PubMed] [Google Scholar]
- Miehlke S., Kibler K., Kim J. G., Figura N., Small S. M., Graham D. Y., Go M. F. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996 Jul;91(7):1322–1325. [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Ogura K., Kanai F., Maeda S., Yoshida H., Ogura M., Lan K. H., Hirota K., Kawabe T., Shiratori Y., Omata M. High prevalence of cytotoxin positive Helicobacter pylori in patients unrelated to the presence of peptic ulcers in Japan. Gut. 1997 Oct;41(4):463–468. doi: 10.1136/gut.41.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. J., van der Hulst R. W., Feller M., Xiao S. D., Tytgat G. N., Dankert J., van der Ende A. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients with peptic ulcer disease and those with chronic gastritis-associated dyspepsia. J Clin Microbiol. 1997 Jun;35(6):1344–1347. doi: 10.1128/jcm.35.6.1344-1347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Phadnis S. H., Ilver D., Janzon L., Normark S., Westblom T. U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect Immun. 1994 May;62(5):1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt W., Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol. 1994 Apr;12(2):307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Shimoyama T., Fukuda S., Tanaka M., Mikami T., Saito Y., Munakata A. High prevalence of the CagA-positive Helicobacter pylori strains in Japanese asymptomatic patients and gastric cancer patients. Scand J Gastroenterol. 1997 May;32(5):465–468. doi: 10.3109/00365529709025082. [DOI] [PubMed] [Google Scholar]
- Tee W., Lambert J. R., Dwyer B. Cytotoxin production by Helicobacter pylori from patients with upper gastrointestinal tract diseases. J Clin Microbiol. 1995 May;33(5):1203–1205. doi: 10.1128/jcm.33.5.1203-1205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford J. L., Ghiara P., Dell'Orco M., Comanducci M., Burroni D., Bugnoli M., Tecce M. F., Censini S., Covacci A., Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994 May 1;179(5):1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Weel J. F., van der Hulst R. W., Gerrits Y., Roorda P., Feller M., Dankert J., Tytgat G. N., van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996 May;173(5):1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]