Abstract
Background/Aims—Infection with Helicobacter pylori strains harbouring the cagA gene (cagA+) is associated with an increased risk of developing peptic ulcer and gastric cancer. The aim of this study was to assess whether H pylori isolates with different cagA status were present in patients with non-ulcer dyspepsia, and whether a variable cagA status is relevant to histological gastric mucosal damage and glandular cell proliferation. Methods—Well separated H pylori colonies (between 2 and 25) from primary plates, per gastric area, for each of 19 patients with non-ulcer dyspepsia were examined for cagA by hybridisation. Western blotting was used to examine both representative colonies for CagA expression and the patients' sera for antibody response to CagA. Glandular gastric cell proliferation was assessed immunohistochemically. Results—Of the 747 colonies examined, 45.3% were cagA+. All colonies from four patients were cagA+, and all colonies from two patients were cagA−. In 13 patients (68%) both cagA+ and cagA− colonies were found. CagA expression of isolates corresponded to their cagA status. H pylori strains with different CagA molecular masses were present in three patients. Results based on all 19patients studied showed that the prevalence of cagA+ colonies in areas with mucosal atrophy associated or not with intestinal metaplasia (67.9%) was significantly higher than in normal mucosa (44.7%) and mucosa from patients with chronic gastritis (44.0%) (p< 0.001). High levels of cell proliferation were associated with histological atrophy with or without intestinal metaplasia, but not with the possession of cagA by organisms colonising the same mucosal sites. Conclusions—Most patients with non-ulcer dyspepsia are infected by both cagA+ and cagA−H pylori colonies. The cagA status of infecting organisms may play a role in the development of atrophy and intestinal metaplasia.
Keywords: gastritis; Helicobacter pylori infection; cagA; mucosal atrophy; cell proliferation
Full Text
The Full Text of this article is available as a PDF (143.6 KB).
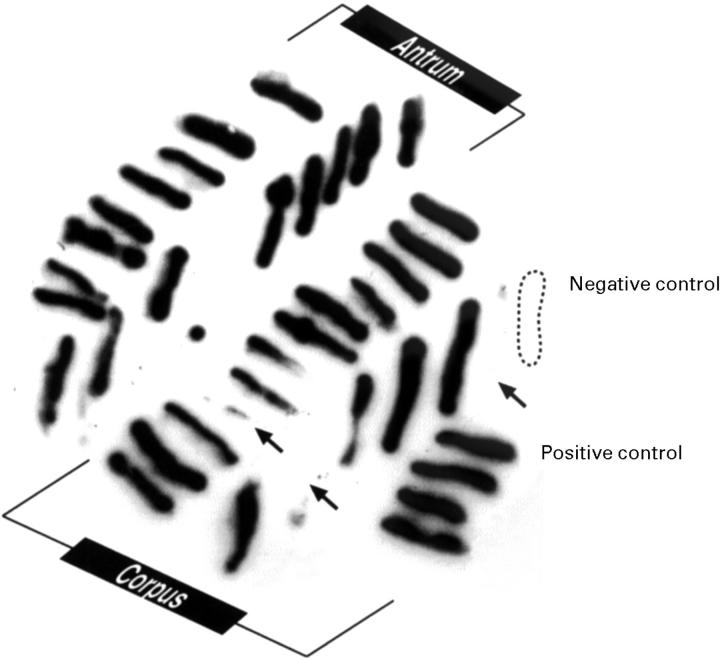
Figure 1 .
Hybridisation with cagA probe of subcultures of different H pylori colonies from primary plates streaked with biopsy samples taken from two gastric areas from the same patient. All 16 colonies from the antrum and 18 of 21 from the corpus are cagA+ (six of 13 colonies from the fundus were cagA+; data not shown). The arrows indicate the cagA− colonies.
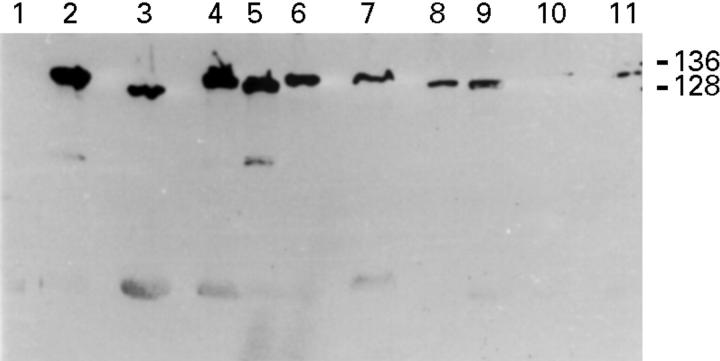
Figure 2 .
Western blots showing , in three cases (lanes 1-3, 4-6, and 7-9), the simultaneous presence of H pylori strains with different CagA molecular mass in different areas of single patients. The strain in lane 1 was cagA−. The strains in lanes 10 and 11 were two cagA+ H pylori strains of similar CagA molecular mass isolated from two different gastric areas of a patient. The numbers on the right are the molecular masses in kDa of H pylori strains CCUG 17874 and G39.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beales I. L., Crabtree J. E., Scunes D., Covacci A., Calam J. Antibodies to CagA protein are associated with gastric atrophy in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1996 Jul;8(7):645–649. [PubMed] [Google Scholar]
- Bechi P., Balzi M., Becciolini A., Maugeri A., Raggi C. C., Amorosi A., Dei R. Helicobacter pylori and cell proliferation of the gastric mucosa: possible implications for gastric carcinogenesis. Am J Gastroenterol. 1996 Feb;91(2):271–276. [PubMed] [Google Scholar]
- Blaser M. J. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987 Aug;93(2):371–383. doi: 10.1016/0016-5085(87)91028-6. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995 May 15;55(10):2111–2115. [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P., Ruiz B., Shi T. Y., Janney A., Sobhan M., Torrado J., Hunter F. Helicobacter pylori and nucleolar organizer regions in the gastric antral mucosa. Am J Clin Pathol. 1994 May;101(5):656–660. doi: 10.1093/ajcp/101.5.656. [DOI] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Fantry G. T., Zheng Q. X., Darwin P. E., Rosenstein A. H., James S. P. Mixed infection with cagA-positive and cagA-negative strains of Helicobacter pylori. Helicobacter. 1996 Jun;1(2):98–106. doi: 10.1111/j.1523-5378.1996.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Azuma T., Murakita H., Hirai M., Miyaji H., Ito Y., Ohtaki Y., Yamazaki Y., Kuriyama M., Keida Y. Profile of Helicobacter pylori cytotoxin derived from two areas of Japan with different prevalence of atrophic gastritis. Gut. 1996 Dec;39(6):800–806. doi: 10.1136/gut.39.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen M., Daskalopoulos G., Warburton V., Mitchell H. M., Hazell S. L. Multiple strain colonization and metronidazole resistance in Helicobacter pylori-infected patients: identification from sequential and multiple biopsy specimens. J Infect Dis. 1996 Sep;174(3):631–635. doi: 10.1093/infdis/174.3.631. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muñoz N., Kato I., Peraza S., Lopez G., Carrillo E., Ramirez H., Vivas J., Castro D., Sanchez V., Andrade O. Prevalence of precancerous lesions of the stomach in Venezuela. Cancer Epidemiol Biomarkers Prev. 1996 Jan;5(1):41–46. [PubMed] [Google Scholar]
- Owen R. J., Desai M., Figura N., Bayeli P. F., Di Gregorio L., Russi M., Musmanno R. A. Comparisons between degree of histological gastritis and DNA fingerprints, cytotoxicity and adhesivity of Helicobacter pylori from different gastric sites. Eur J Epidemiol. 1993 May;9(3):315–321. doi: 10.1007/BF00146270. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Orentreich N., Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997 Mar;40(3):297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Replogle M., Yang S., Hiatt R. Seroprevalence of CagA-positive strains among Helicobacter pylori-infected, healthy young adults. J Infect Dis. 1997 May;175(5):1240–1242. doi: 10.1086/593680. [DOI] [PubMed] [Google Scholar]
- Phadnis S. H., Ilver D., Janzon L., Normark S., Westblom T. U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect Immun. 1994 May;62(5):1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci V., Ciacci C., Zarrilli R., Sommi P., Tummuru M. K., Del Vecchio Blanco C., Bruni C. B., Cover T. L., Blaser M. J., Romano M. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996 Jul;64(7):2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Fox J. G., Akopyants N. S., Berg D. E., Thompson N., Shames B., Yan L., Fontham E., Janney F., Hunter F. M. Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. J Clin Microbiol. 1995 Apr;33(4):918–923. doi: 10.1128/jcm.33.4.918-923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weel J. F., van der Hulst R. W., Gerrits Y., Roorda P., Feller M., Dankert J., Tytgat G. N., van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996 May;173(5):1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- Wotherspoon A. C., Ortiz-Hidalgo C., Falzon M. R., Isaacson P. G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991 Nov 9;338(8776):1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., Rauws E. A., Feller M., Mulder C. J., Tytgat G. N., Dankert J. Heterogeneous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996 Sep;111(3):638–647. doi: 10.1053/gast.1996.v111.pm8780568. [DOI] [PubMed] [Google Scholar]