Abstract
Background—Partial obstruction of the ileum causes a notable hypertrophy of smooth muscle cells and enteric neurones in the proximally located intestine. Aims—To study the expression of neuromessengers in the hypertrophic ileum of rat as little is known about neuromessenger plasticity under these conditions. To investigate the presence of interstitial cells of Cajal (ICC) in hypertrophic ileum. Methods—Ileal hypertrophy was induced by circumferential application of a strip of plastic film for 18-24 days. Immunocytochemistry, in situ hybridisation, nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase histochemistry, and ethidium bromide staining were used to investigate the number of enteric neurones expressing neuropeptides and nitric oxide synthase, and the frequency of ICC. Results—In the hypertrophic ileum several neuronal populations showed changes in their expression of neuromessengers. Myenteric neurones expressing vasoactive intestinal peptide (VIP), pituitary adenylate cyclase activating peptide, and galanin were notably increased in number. In submucous ganglia the number of VIP immunoreactive neurones decreased while those expressing VIP mRNA increased. NADPH diaphorase positive submucous neurones increased dramatically while the number of neuronal type nitric oxide synthase expressing ones was unchanged. The number of ICC decreased notably in hypertrophic ileum. Conclusion—Enteric neurones change their levels of expression of neuromessengers in hypertrophic ileum. ICC are also affected. The changes are presumably part of an adaptive response to the increased work load.
Keywords: enteric nerves; interstitial cells of Cajal; hypertrophy; neuropeptides; nitric oxide; neuronal plasticity
Full Text
The Full Text of this article is available as a PDF (228.0 KB).
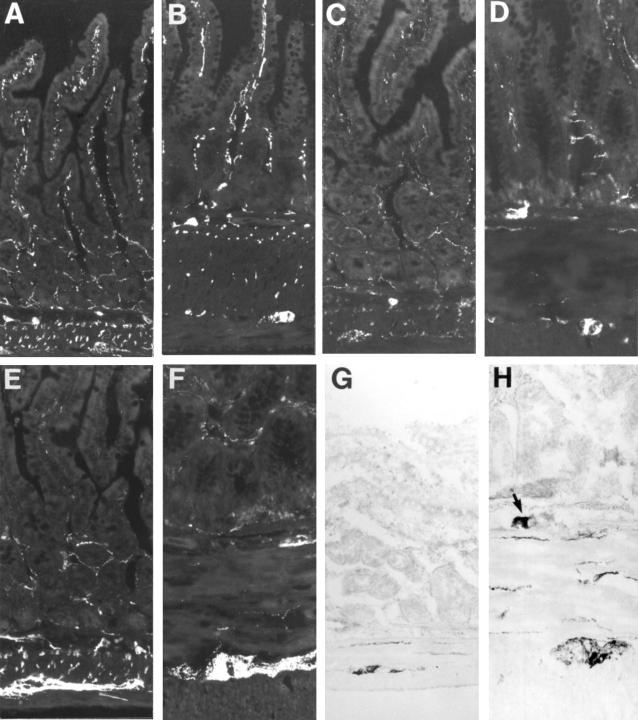
Figure 1 .
Cryostat sections from rat: control (A,C,E,G) and hypertrophic (B,D,F,H) ileum, immunostained for VIP (A,B), PACAP (C,D), and galanin (E,F) or NADPH diaphorase stained (G,H). The notable thickening of the smooth muscle layers in hypertrophic ileum and the decreased frequency of nerve fibres in these layers are obvious. The size of the neuronal cell bodies is increased in hypertrophic ileum. Note in (H) a large NADPH diaphorase positive nerve cell body within the submucosal ganglion (arrow); such cell bodies are commonly seen in hypertrophic ileum but are extremely rare in control tissue. Original magnification ×130.
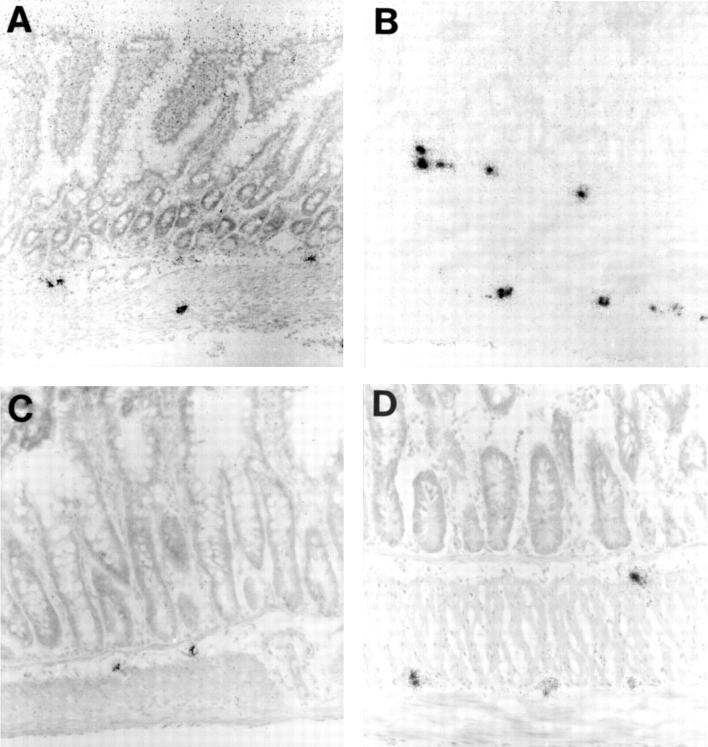
Figure 2 .
Cryostat sections from rat: control (A,C) and hypertrophic (B,D) ileum autoradiographically labelled for VIP mRNA (A,B) and galanin mRNA (C,D). VIP mRNA containing nerve cell bodies are larger, more numerous, and more intensely labelled in hypertrophic ileum. The number of galanin mRNA containing nerve cell bodies is particularly increased in the myenteric ganglia where they are larger and more intensely labelled. Original magnification ×130.
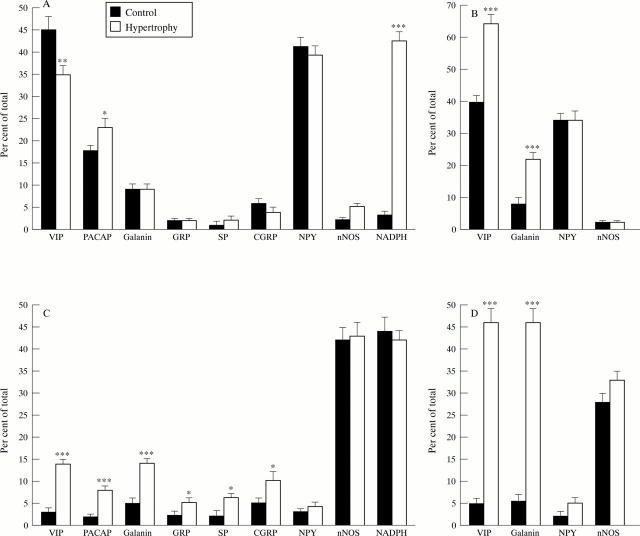
Figure 3 .
Numbers of VIP, PACAP, galanin, GRP, SP, CGRP, NPY, and nNOS immunoreactive, NADPH diaphorase positive (A,C), and VIP mRNA, galanin mRNA, NPY mRNA, and nNOS mRNA expressing (B,D) neuronal cell bodies in submucosal (A,B) and myenteric ganglia (C,D) from control and hypertrophic rat ileum. The numbers of positive cell bodies are expressed as the percentage of the total number of cell bodies, established by ethidium bromide staining. Mean (SEM), *p<0.05, **p<0.01, ***p<0.001.
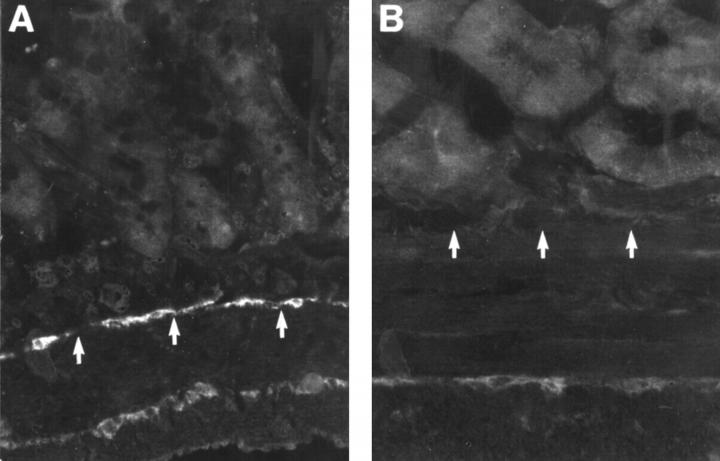
Figure 4 .
Cryostat sections from rat: control (A) and hypertrophic (B) ileum, showing the presence of ICC using antiserum against c-kit receptor. Numerous ICC can be found within the deep muscular plexus and at the border of longitudinal and circular muscle in the control (A). In the hypertrophic ileum (B) ICC are notably reduced in number, particularly in the deep muscular plexus. The positions of the deep muscular plexus are indicated by arrows. Original magnification ×160.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Burns A. J., Lomax A. E., Torihashi S., Sanders K. M., Ward S. M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J., Rick G. A. Interstitial cells of Cajal in the rat colon are damaged by mild hypoxia. J Auton Nerv Syst. 1994 Jul;48(2):175–180. doi: 10.1016/0165-1838(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Earlam R. J. Ganglion cell changes in experimental stenosis of the gut. Gut. 1971 May;12(5):393–398. doi: 10.1136/gut.12.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E., Alm P., Sundler F. Distribution, origin and projections of nitric oxide synthase-containing neurons in gut and pancreas. Neuroscience. 1994 Nov;63(1):233–248. doi: 10.1016/0306-4522(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Ekman R., Håkanson R., Sundler F. Projections of peptide-containing neurons in rat colon. Neuroscience. 1988 Nov;27(2):655–674. doi: 10.1016/0306-4522(88)90296-5. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Mulder H., Sundler F. Vasoactive intestinal peptide expression in enteric neurons is upregulated by both colchicine and axotomy. Regul Pept. 1996 Jul 5;63(2-3):113–121. doi: 10.1016/0167-0115(96)00028-6. [DOI] [PubMed] [Google Scholar]
- Ekblad E., Winther C., Ekman R., Håkanson R., Sundler F. Projections of peptide-containing neurons in rat small intestine. Neuroscience. 1987 Jan;20(1):169–188. doi: 10.1016/0306-4522(87)90010-8. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Young H. M., Pompolo S., Bornstein J. C., Kunze W. A., McConalogue K. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology. 1995 Feb;108(2):554–563. doi: 10.1016/0016-5085(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Gabella G. Hypertrophy of intestinal smooth muscle. Cell Tissue Res. 1975 Nov 7;163(2):199–214. [PubMed] [Google Scholar]
- Gabella G. Hypertrophy of visceral smooth muscle. Anat Embryol (Berl) 1990;182(5):409–424. doi: 10.1007/BF00178906. [DOI] [PubMed] [Google Scholar]
- Gabella G. Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea-pig. J Neurocytol. 1984 Feb;13(1):73–84. doi: 10.1007/BF01148319. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Zhang X., Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994 Jan;17(1):22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Jew J. Y., Williams T. H., Gabella G., Zhang M. Q. The intestine as a model for neuronal plasticity. Arch Histol Cytol. 1989;52 (Suppl):167–180. doi: 10.1679/aohc.52.suppl_167. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Spindel E. R., Isselbacher K. J., Chin W. W. Tissue-specific expression of the rat galanin gene. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1065–1069. doi: 10.1073/pnas.85.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S. The centenary of the problem of the interstitial cells of Cajal. Kaibogaku Zasshi. 1996 Dec;71(6):629–637. [PubMed] [Google Scholar]
- Komuro T., Zhou D. S. Anti-c-kit protein immunoreactive cells corresponding to the interstitial cells of Cajal in the guinea-pig small intestine. J Auton Nerv Syst. 1996 Nov 6;61(2):169–174. doi: 10.1016/s0165-1838(96)00078-1. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Ericsson A., Persson H. Structure and expression of the rat neuropeptide Y gene. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2068–2072. doi: 10.1073/pnas.84.7.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Skaper S. D., Dal Toso R., Petrelli L., Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996 Nov;19(11):514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- Magnusson S., Alm P., Kanje M. Inducible nitric oxide synthase increases in regenerating rat ganglia. Neuroreport. 1996 Aug 12;7(12):2046–2050. doi: 10.1097/00001756-199608120-00039. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Thompson J. H., Zhang X. J., Sadowska-Krowicka H., Kakkis J. L., Munshi U. K., Sandoval M., Rossi J. L., Eloby-Childress S., Beckman J. S. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995 Nov;109(5):1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- Minc-Golomb D., Tsarfaty I., Schwartz J. P. Expression of inducible nitric oxide synthase by neurones following exposure to endotoxin and cytokine. Br J Pharmacol. 1994 Jul;112(3):720–722. doi: 10.1111/j.1476-5381.1994.tb13136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mulder H., Uddman R., Moller K., Zhang Y. Z., Ekblad E., Alumets J., Sundler F. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994 Nov;63(1):307–312. doi: 10.1016/0306-4522(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Hayakawa Y., Yanaihara N., Okamoto H. Nucleotide sequence divergence and functional constraint in VIP precursor mRNA evolution between human and rat. FEBS Lett. 1985 Apr 8;183(1):55–59. doi: 10.1016/0014-5793(85)80953-4. [DOI] [PubMed] [Google Scholar]
- Saffrey M. J., Burnstock G. Growth factors and the development and plasticity of the enteric nervous system. J Auton Nerv Syst. 1994 Nov;49(3):183–196. doi: 10.1016/0165-1838(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Sanders K. M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996 Aug;111(2):492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Schmued L. C., Swanson L. W., Sawchenko P. E. Some fluorescent counterstains for neuroanatomical studies. J Histochem Cytochem. 1982 Feb;30(2):123–128. doi: 10.1177/30.2.6174560. [DOI] [PubMed] [Google Scholar]
- Sundler F., Ekblad E., Absood A., Håkanson R., Köves K., Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46(2):439–454. doi: 10.1016/0306-4522(92)90064-9. [DOI] [PubMed] [Google Scholar]
- Sundler F., Ekblad E., Hannibal J., Moller K., Zhang Y. Z., Mulder H., Elsås T., Grunditz T., Danielsen N., Fahrenkrug J. Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann N Y Acad Sci. 1996 Dec 26;805:410–428. doi: 10.1111/j.1749-6632.1996.tb17501.x. [DOI] [PubMed] [Google Scholar]
- Vanderwinden J. M., Liu H., De Laet M. H., Vanderhaeghen J. J. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996 Aug;111(2):279–288. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- Williams T. H., Zhang M. Q., Jew J. Y. Hypertrophy of rat sensory ganglion neurons following intestinal obstruction. Gastroenterology. 1993 Jul;105(1):8–14. doi: 10.1016/0016-5085(93)90004-v. [DOI] [PubMed] [Google Scholar]