Abstract
Background—The development of clinical disease after infection with Helicobacter pylori has been reported to be associated with expression of the cagA gene. Recently, it has been shown that cagA is part of a multigene locus, described as the cag pathogenicity island (PAI). The role of this region in determining clinical outcome remains to be established. Aims—To investigate whether the presence of cagA is always associated with the presence of the complete cag PAI and to evaluate the distribution of selected cag genes in 73 H pylori strains isolated from patients in France. Methods—Clinical strains of H pylori were screened for selected genes of the cag PAI by polymerase chain reaction and colony hybridisation. Results—Of 64 strains that harboured the cagA gene, 57 (89%) also contained the entire cag PAI. The entire cag PAI was found in 85% (48/56) and 53% (9/17) of duodenal ulcer and non-ulcer dyspepsia isolates, respectively. Eight strains had deletions within the cag PAI, including deletion of the cagA gene in one isolate; the deletions were not associated with the insertion sequence IS605. Of eight strains lacking the cag PAI, four were isolated from patients with duodenal ulcer. Conclusion—The cag PAI is not a uniform, conserved entity. Although the presence of the cag PAI is highly associated with duodenal ulcer, the clinical outcome of infection with H pylori is not reliably predicted by any gene of the cag PAI.
Keywords: duodenal ulcer; Helicobacter pylori; cag pathogenicity island; non-ulcer dyspepsia.
Full Text
The Full Text of this article is available as a PDF (143.1 KB).
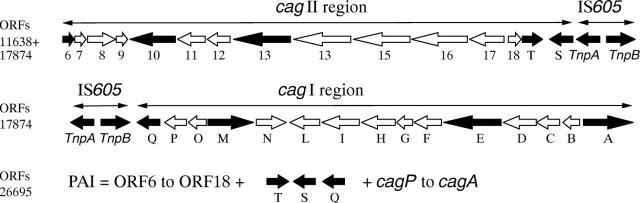
Figure 1 .
Schematic representation of the cag pathogenicity island of H pylori as previously published for the strains NCTC 11638, CCUG 17874, and 26695.2627 Strain 26695 contains a single, contiguous cag PAI, whereas in strain CCUG 17874 (also designated NCTC 11638) the PAI is divided into two regions, cagI and cagII, by intervening chromosomal DNA and the insertion sequence IS605.2627 Arrows represent predicted open reading frames; shaded arrows represent genes targeted in this study.
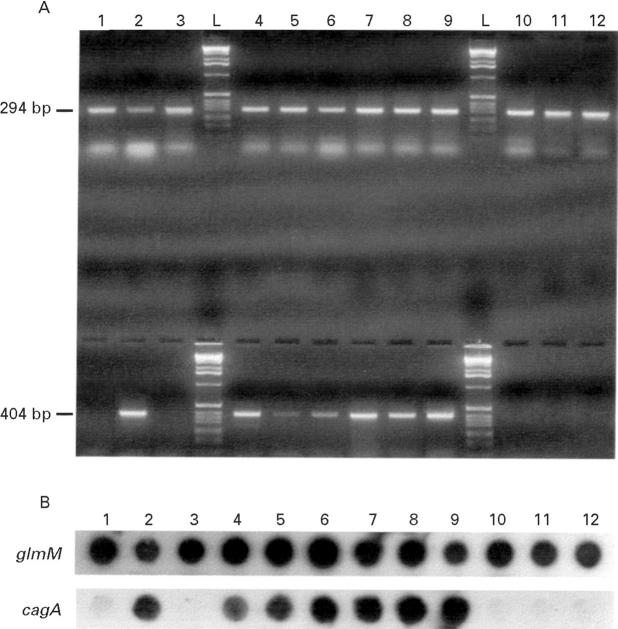
Figure 2 .
(A) Example of detection of PCR products by agarose gel electrophoresis and ethidium bromide staining. Strains were initially tested for the presence of glmM (upper lanes) and cagA (lower lanes). Lanes 1 to 12 represent strains 26 to 37; lanes L contain a molecular weight marker (Gibco/BRL Ltd, Paisley, UK). (B) Representative colony hybridisation to detect the presence of glmM and cagA. Dots 1 to 12 represent strains 26 to 37. The photocomposition of the figure was obtained from the original Polaroid film plus the autoradiographs from the colony hybridisations with a Studioscan IIsi scanner (AGFA, Mortsel, Belgium). After the initial image was scanned and saved as a PICT file, the file was opened in Adobe Photoshop, version 3.0 (Adobe system Inc. Mountain View, California, USA).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel I., Jacobs E., Kist M., Bredt W. Antibody response of patients against a 120 kDa surface protein of Campylobacter pylori. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):271–276. doi: 10.1016/s0176-6724(88)80012-9. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori: microbiology of a 'slow' bacterial infection. Trends Microbiol. 1993 Oct;1(7):255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995 May 15;55(10):2111–2115. [PubMed] [Google Scholar]
- Blaser M. J. Role of vacA and the cagA locus of Helicobacter pylori in human disease. Aliment Pharmacol Ther. 1996 Apr;10 (Suppl 1):73–77. doi: 10.1046/j.1365-2036.1996.22164008.x. [DOI] [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Falkow S., Berg D. E., Rappuoli R. Did the inheritance of a pathogenicity island modify the virulence of Helicobacter pylori? Trends Microbiol. 1997 May;5(5):205–208. doi: 10.1016/S0966-842X(97)01035-4. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Glupczynski Y., Lage A. P., Burette A., Tummuru M. K., Perez-Perez G. I., Blaser M. J. Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol. 1995 Jun;33(6):1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Covacci A., Farmery S. M., Xiang Z., Tompkins D. S., Perry S., Lindley I. J., Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995 Jan;48(1):41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Xiang Z., Lindley I. J., Tompkins D. S., Rappuoli R., Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995 Oct;48(10):967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazek E. S., Dubois A., Holmes R. K., Kersulyte D., Akopyants N. S., Berg D. E., Warren R. L. Cloning and characterization of hemolytic genes from Helicobacter pylori. Infect Immun. 1995 Nov;63(11):4345–4349. doi: 10.1128/iai.63.11.4345-4349.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Genta R. M., Graham D. P., Crabtree J. E. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: lack of correlation of IgG antibody in patients with peptic ulcer or asymptomatic Helicobacter pylori gastritis. J Clin Pathol. 1996 Oct;49(10):829–832. doi: 10.1136/jcp.49.10.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen M., Janatuinen E., Mayo K., Mégraud F., Julkunen R., Pikkarainen P. Usefulness of anti-Helicobacter pylori and anti-CagA antibodies in the selection of patients for gastroscopy. Am J Gastroenterol. 1997 Dec;92(12):2225–2229. [PubMed] [Google Scholar]
- Heikkinen M., Mayo K., Mégraud F., Vornanen M., Marin S., Pikkarainen P., Julkunen R. Association of CagA-positive and CagA-negative Helicobacter pylori strains with patients' symptoms and gastritis in primary care patients with functional upper abdominal complaints. Scand J Gastroenterol. 1998 Jan;33(1):31–38. doi: 10.1080/00365529850166176. [DOI] [PubMed] [Google Scholar]
- Hu P. J., Li Y. Y., Zhou M. H., Chen M. H., Du G. G., Huang B. J., Mitchell H. M., Hazell S. L. Helicobacter pylori associated with a high prevalence of duodenal ulcer disease and a low prevalence of gastric cancer in a developing nation. Gut. 1995 Feb;36(2):198–202. doi: 10.1136/gut.36.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaswal R. K., Veluthambi K., Gelvin S. B., Slightom J. L. Double-stranded cleavage of T-DNA and generation of single-stranded T-DNA molecules in Escherichia coli by a virD-encoded border-specific endonuclease from Agrobacterium tumefaciens. J Bacteriol. 1987 Nov;169(11):5035–5045. doi: 10.1128/jb.169.11.5035-5045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansau I., Raymond J., Bingen E., Courcoux P., Kalach N., Bergeret M., Braimi N., Dupont C., Labigne A. Genotyping of Helicobacter pylori isolates by sequencing of PCR products and comparison with the RAPD technique. Res Microbiol. 1996 Oct;147(8):661–669. doi: 10.1016/0923-2508(96)84023-x. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L. L. Genetic and molecular characterization of the polar flagellum of Vibrio parahaemolyticus. J Bacteriol. 1995 Mar;177(6):1595–1609. doi: 10.1128/jb.177.6.1595-1609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Orentreich N., Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997 Mar;40(3):297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Hansen S., Rodriguez L., Gelb A. B., Warnke R. A., Jellum E., Orentreich N., Vogelman J. H., Friedman G. D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994 May 5;330(18):1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- Segal E. D., Falkow S., Tompkins L. S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E. D., Lange C., Covacci A., Tompkins L. S., Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. A., Tummuru M. K., Miller G. G., Blaser M. J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995 May;63(5):1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleischmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997 Aug 7;388(6642):539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Mutation of the cytotoxin-associated cagA gene does not affect the vacuolating cytotoxin activity of Helicobacter pylori. Infect Immun. 1994 Jun;62(6):2609–2613. doi: 10.1128/iai.62.6.2609-2613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. E., Akiyoshi D. E., Regier D., Datta A., Gordon M. P., Nester E. W. Characterization of the virB operon from an Agrobacterium tumefaciens Ti plasmid. J Biol Chem. 1988 Apr 25;263(12):5804–5814. [PubMed] [Google Scholar]
- Weel J. F., van der Hulst R. W., Gerrits Y., Roorda P., Feller M., Dankert J., Tytgat G. N., van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996 May;173(5):1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., Rauws E. A., Feller M., Mulder C. J., Tytgat G. N., Dankert J. Heterogeneous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996 Sep;111(3):638–647. doi: 10.1053/gast.1996.v111.pm8780568. [DOI] [PubMed] [Google Scholar]