Abstract
Background—Despite being immunogenic, gastric cancers overcome antitumour immune responses by mechanisms that have yet to be fully elucidated. Fas ligand (FasL) is a molecule that induces Fas receptor mediated apoptosis of activated immunocytes, thereby mediating normal immune downregulatory roles including immune response termination, tolerance acquisition, and immune privilege. Colon cancer cell lines have previously been shown to express FasL and kill lymphoid cells by Fas mediated apoptosis in vitro. Many diverse tumours have since been found to express FasL suggesting that a "Fas counterattack" against antitumour immune effector cells may contribute to tumour immune escape. Aim—To ascertain if human gastric tumours express FasL in vivo, as a potential mediator of immune escape in stomach cancer. Specimens—Thirty paraffin wax embedded human gastric adenocarcinomas. Methods—FasL protein was detected in gastric tumours using immunohistochemistry; FasL mRNA was detected in the tumours using in situ hybridisation. Cell death was detected in situ in tumour infiltrating lymphocytes using terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL). Results—Prevalent expression of FasL was detected in all 30 resected gastric adenocarcinomas examined. In the tumours, FasL protein and mRNA were co-localised to neoplastic gastric epithelial cells, confirming expression by the tumour cells. FasL expression was independent of tumour stage, suggesting that it may be expressed throughout gastric cancer progression. TUNEL staining disclosed a high level of cell death among lymphocytes infiltrating FasL positive areas of tumour. Conclusions—Human gastric adenocarcinomas express the immune downregulatory molecule, FasL. The results suggest that FasL is a prevalent mediator of immune privilege in stomach cancer.
Keywords: Fas ligand; gastric cancer; immune escape; apoptosis; tumour; mRNA
Full Text
The Full Text of this article is available as a PDF (223.5 KB).
Figure 1 .
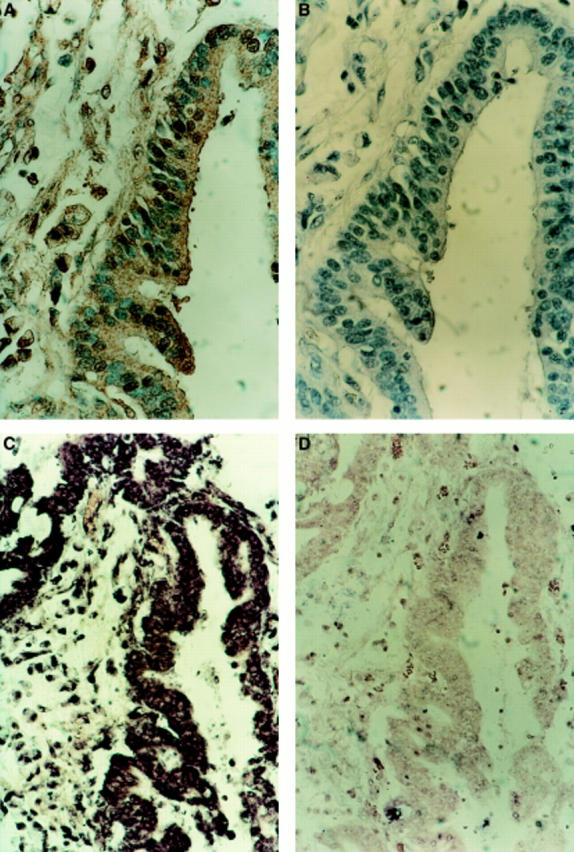
Expression of Fas ligand (FasL) in human gastric adenocarcinomas. Immunoperoxidase staining using a FasL specific rabbit polyclonal IgG was performed on paraffin wax embedded gastric carcinoma sections. Slides were counterstained with haematoxylin. (A) FasL positive immunohistochemical staining (brown) is shown in a representative gastric adenocarcinoma. (B) As a control for specificity of antibody detection, the FasL immunising peptide was included during primary antibody incubation. Competitive displacement of staining by the immunising peptide confirms FasL specificity. Tumour sections sequential to those shown in (A) and (B) were used to detect FasL mRNA by in situ hybridisation, using a digoxigenin labelled FasL specific riboprobe. (C) Positive purple hybridisation signal was obtained from the tumour area that stained immunohistochemically positive for FasL protein. (D) In a control hybridisation, a tenfold excess of unlabelled probe caused direct competitive displacement of the labelled probe, confirming the specificity of hybridisation. These results are representative of 30 adenocarcinomas of the stomach.
Figure 2 .
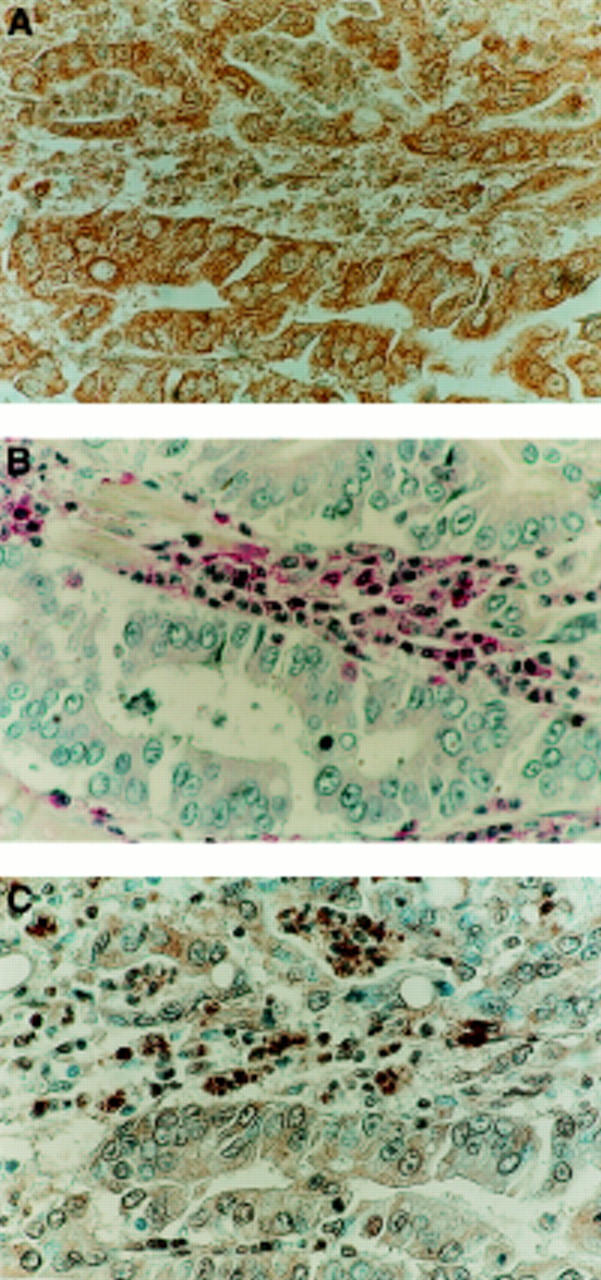
Apoptotic lymphocytes adjacent to FasL expressing carcinoma. (A) FasL expression (brown) detected in an area of gastric carcinoma by immunoperoxidase staining. (B) CD45 (leucocyte common antigen) immunophosphatase (alkaline phosphatase conjugated anti-alkaline phosphatase (APAAP)) staining (red) was performed on a sequential section. CD45 positive cells (red) of lymphoid morphology are present adjacent to areas of carcinoma. Slides were counterstained with haematoxylin. Isotype matched control sections were negative (not shown). (C) Cell death detection in situ by terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL). Tumour sections sequential to those shown in (A) and (B) were used to detect cell death by enzymic labelling of DNA strand breaks using TUNEL. Only those cells with positive TUNEL staining (brown) and exhibiting apoptotic morphology were considered apoptotic. Control sections (not shown) where the labelling enzyme was omitted were negative.
Figure 3 .
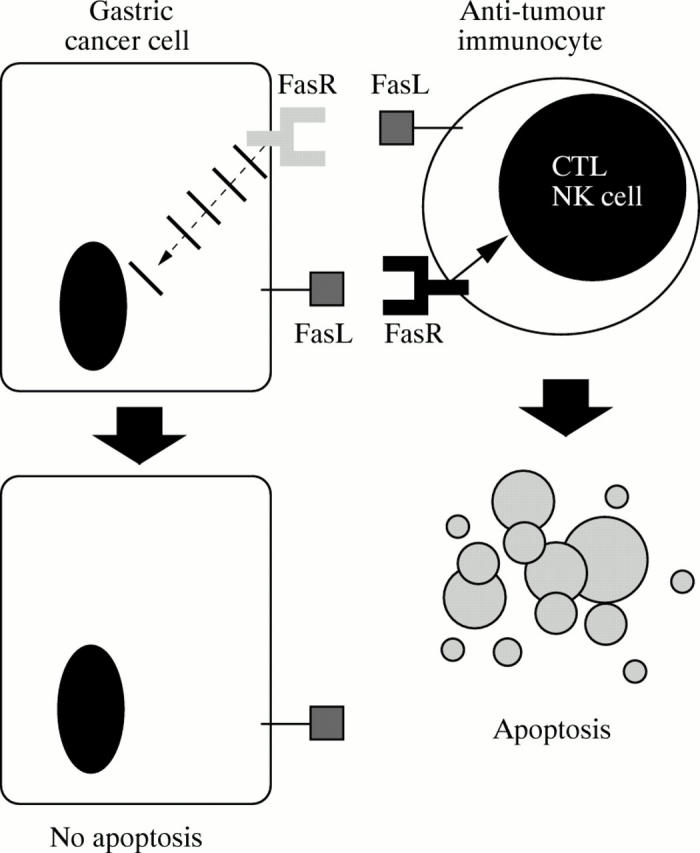
The Fas counterattack. By expressing functional Fas ligand (FasL), gastric cancer cells can potentially counterattack and cause apoptosis of Fas sensitive tumour infiltrating immunocytes, which include antitumour T (CTL) and NK cells. FasR, Fas receptor. As the result of various acquired defects in Fas signal transduction, most tumours are themselves relatively resistant to FasL mediated apoptosis.23
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Tough T. W., Davis-Smith T., Braddy S., Falk B., Schooley K. A., Goodwin R. G., Smith C. A., Ramsdell F., Lynch D. H. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995 Jan 1;181(1):71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J., Georgiou H. M., Strasser A., Vaux D. L. Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H., Chan S. Y., Bishop D. K., Nabel G. J. Inhibition of the alloantibody response by CD95 ligand. Nat Med. 1997 Aug;3(8):843–848. doi: 10.1038/nm0897-843. [DOI] [PubMed] [Google Scholar]
- Arai H., Gordon D., Nabel E. G., Nabel G. J. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13862–13867. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrau D., Gold D., Selawry H., Moore J., Franzusoff A., Duke R. C. A role for CD95 ligand in preventing graft rejection. Nature. 1995 Oct 19;377(6550):630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. W., O'Connell J., O'Sullivan G. C., Brady C., Roche D., Collins J. K., Shanahan F. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998 Jun 1;160(11):5669–5675. [PubMed] [Google Scholar]
- Daniel P. T., Krammer P. H. Activation induces sensitivity toward APO-1 (CD95)-mediated apoptosis in human B cells. J Immunol. 1994 Jun 15;152(12):5624–5632. [PubMed] [Google Scholar]
- Eischen C. M., Schilling J. D., Lynch D. H., Krammer P. H., Leibson P. J. Fc receptor-induced expression of Fas ligand on activated NK cells facilitates cell-mediated cytotoxicity and subsequent autocrine NK cell apoptosis. J Immunol. 1996 Apr 15;156(8):2693–2699. [PubMed] [Google Scholar]
- Galle P. R., Hofmann W. J., Walczak H., Schaller H., Otto G., Stremmel W., Krammer P. H., Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995 Nov 1;182(5):1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995 Nov 17;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Hahne M., Rimoldi D., Schröter M., Romero P., Schreier M., French L. E., Schneider P., Bornand T., Fontana A., Lienard D. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996 Nov 22;274(5291):1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Seki N., Kikuchi M., Kuramoto T., Iwamoto O., Kodama I., Koufuji K., Takeda J., Itoh K. HLA class-I-restricted and tumor-specific CTL in tumor-infiltrating lymphocytes of patients with gastric cancer. Int J Cancer. 1997 Mar 17;70(6):631–638. doi: 10.1002/(sici)1097-0215(19970317)70:6<631::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hunt J. S., Vassmer D., Ferguson T. A., Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997 May 1;158(9):4122–4128. [PubMed] [Google Scholar]
- Inoue H., Mori M., Honda M., Li J., Shibuta K., Mimori K., Ueo H., Akiyoshi T. The expression of tumor-rejection antigen "MAGE" genes in human gastric carcinoma. Gastroenterology. 1995 Nov;109(5):1522–1525. doi: 10.1016/0016-5085(95)90639-8. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Schneider D. B., Lin Z., Hanahan D., Dichek D. A., Stock P. G., Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997 Jul;3(7):738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- Korbutt G. S., Elliott J. F., Rajotte R. V. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997 Feb;46(2):317–322. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- Lau H. T., Stoeckert C. J. FasL--too much of a good thing? Transplanted grafts of pancreatic islet cells engineered to express Fas ligand are destroyed not protected by the immune system. Nat Med. 1997 Jul;3(7):727–728. doi: 10.1038/nm0797-727. [DOI] [PubMed] [Google Scholar]
- Lau H. T., Yu M., Fontana A., Stoeckert C. J., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996 Jul 5;273(5271):109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- Liles W. C., Kiener P. A., Ledbetter J. A., Aruffo A., Klebanoff S. J. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996 Aug 1;184(2):429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara Y., Tomisaki S., Oda S., Kakeji Y., Tsujitani S., Ichiyoshi Y., Akazawa K., Sugimachi K. Lymph node metastasis and relation to tumor growth potential and local immune response in advanced gastric cancer. Int J Cancer. 1997 Apr 22;74(2):224–228. doi: 10.1002/(sici)1097-0215(19970422)74:2<224::aid-ijc15>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Mountz J. D., Zhou T., Bluethmann H., Wu J., Edwards C. K., 3rd Apoptosis defects analyzed in TcR transgenic and fas transgenic lpr mice. Int Rev Immunol. 1994;11(4):321–342. doi: 10.3109/08830189409051178. [DOI] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Niehans G. A., Brunner T., Frizelle S. P., Liston J. C., Salerno C. T., Knapp D. J., Green D. R., Kratzke R. A. Human lung carcinomas express Fas ligand. Cancer Res. 1997 Mar 15;57(6):1007–1012. [PubMed] [Google Scholar]
- O'Connell J., Bennett M. W., O'Sullivan G. C., Collins J. K., Shanahan F. The Fas counterattack: a molecular mechanism of tumor immune privilege. Mol Med. 1997 May;3(5):294–300. [PMC free article] [PubMed] [Google Scholar]
- O'Connell J., O'Sullivan G. C., Collins J. K., Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996 Sep 1;184(3):1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner A. A., Grimm E. A., Lotze M. T., Chu E. W., Rosenberg S. A. Lymphokine-activated killer (LAK) cells. Analysis of factors relevant to the immunotherapy of human cancer. Cancer. 1985 Mar 15;55(6):1327–1333. doi: 10.1002/1097-0142(19850315)55:6<1327::aid-cncr2820550628>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Saas P., Walker P. R., Hahne M., Quiquerez A. L., Schnuriger V., Perrin G., French L., Van Meir E. G., de Tribolet N., Tschopp J. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest. 1997 Mar 15;99(6):1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino K., Kayagaki N., Okumura K., Yagita H. Antitumor effect of locally produced CD95 ligand. Nat Med. 1997 Feb;3(2):165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Tsuji N., Shioda T., Isselbacher K. J., Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand S., Hofmann W. J., Hug H., Müller M., Otto G., Strand D., Mariani S. M., Stremmel W., Krammer P. H., Galle P. R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells--a mechanism of immune evasion? Nat Med. 1996 Dec;2(12):1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Griffith T. S., Usui N., Pepose J., Yu X., Ferguson T. A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997 Feb 1;99(3):396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Suda T., Takahashi T., Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995 Mar 15;14(6):1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Yang Y., Horton J. L., Samoilova E. B., Judge T. A., Turka L. A., Wilson J. M., Chen Y. Amelioration of collagen-induced arthritis by CD95 (Apo-1/Fas)-ligand gene transfer. J Clin Invest. 1997 Oct 15;100(8):1951–1957. doi: 10.1172/JCI119726. [DOI] [PMC free article] [PubMed] [Google Scholar]


