Abstract
BACKGROUND—cag pathogenicity island (PAI) is reported to be a major virulence factor of Helicobacter pylori. AIM—To characterise cagA and the cag PAI in Japanese H pylori strains. METHODS—H pylori isolates from Japanese patients were evaluated for CagA by immunoblot, for cagA transcription by northern blot, and for cagA and 13 other cag PAI genes by Southern blot. cagA negative strains from Western countries were also studied. Induction of interleukin-8 secretion from gastric epithelial cells was also investigated. RESULTS—All Japanese strains retained cagA. Fifty nine of 63 (94%) strains had all the cag PAI genes. In the remaining four, cag PAI was partially deleted, lacking cagA transcripts and not producing CagA protein. Details of the PAI of these strains were checked; three lacked cagB to cagQ (cagI) and continuously cagS to cag13 (cagII), and the remaining one lacked cagB to cag8. Western cagA negative strains completely lacked cag PAI including cagA. Nucleotide sequence analysis in one strain in which the cag PAI was partially deleted showed that the partial deletion contained 25 kb of cag PAI and the cagA promoter. Interleukin-8 induction was lower with the cag PAI partial deletion strains than with the intact ones. All Japanese cag PAI deleted strains were derived from patients with non-ulcer dyspepsia, whereas 41of 59 (70%) CagA-producing strains were from patients with peptic ulcers or gastric cancer (p<0.05). CONCLUSIONS—Most Japanese H pylori strains had the intact cag PAI. However, some lacked most of the cag PAI in spite of the presence of cagA. Thus the presence of the cagA gene is not an invariable marker of cag PAI related virulence in Japanese strains.
Keywords: Helicobacter pylori; pathogenicity island; Japanese
Full Text
The Full Text of this article is available as a PDF (136.9 KB).
Figure 1 .
Map of the cag pathogenicity island. The names of the genes are from GenBank accession number AC000108 and U60176. Cross bars indicate probes used in this study. Fifty nine of 63 strains isolated in Japan all had cagI and cagII. Large deletions were revealed in the remaining four. cagA negative strains isolated in Western countries lacked whole cagI and cagII.
Figure 2 .

Northern blot analysis of cagA transcripts from seven strains. cagA transcripts were present in T-57 (lane 2), T-1 (lane 5), and ATCC 43526 (lane 7), and absent from T-94 (lane 1), T-85, T-25 (lanes 3 and 4), and Tx30a (lane 6). The membrane was also hybridised to 23S rRNA to monitor the amount of RNA loaded.
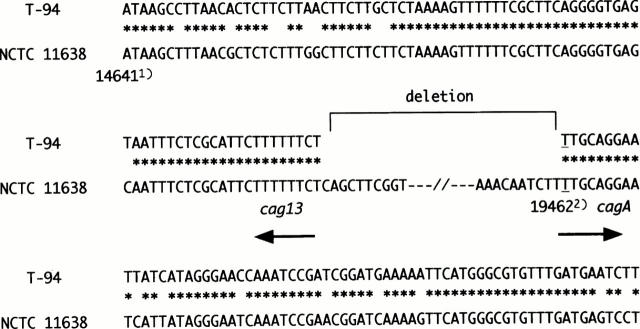
Figure 3 .
Junctional sequences of T-94, which has cag PAI deleted. cagII was present down to position 14 723 (GenBank accession number AC000108) in the middle of cag13. The cagA gene was present up to position 19 462 (GenBank accession number U60176). This position is equal to position 500 of the cagA gene. An approximately 25 kb segment of cag PAI was deleted. 1) indicates the position of GenBank accession number AC000108. 2) indicates the cagA position of GenBank accession number U60176. Asterisks denote identity.
Figure 4 .
Secretion of interleukin-8 (IL-8) from MKN-28 cells over the 16 hours after stimulation. T-77, T-78, T-79, T-81, T-82, T-90, T-100, T-102, T-103, T-108 strains were clinical isolates with an intact cag pathogenicity island (PAI). 43579 indicates ATCC 43579. Tx30a is a strain with cag PAI completely deleted. In T-25, T-68, T-85, and T-94, cag PAI was partially deleted. Results are expressed as the mean and SD from four to six experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyants N. S., Clifton S. W., Kersulyte D., Crabtree J. E., Youree B. E., Reece C. A., Bukanov N. O., Drazek E. S., Roe B. A., Berg D. E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998 Apr;28(1):37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995 May 15;55(10):2111–2115. [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C. K., Wong B. C., Kwok E., Ong L., Covacci A., Lam S. K. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996 May;91(5):949–953. [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Glupczynski Y., Lage A. P., Burette A., Tummuru M. K., Perez-Perez G. I., Blaser M. J. Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol. 1995 Jun;33(6):1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Covacci A., Farmery S. M., Xiang Z., Tompkins D. S., Perry S., Lindley I. J., Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995 Jan;48(1):41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Forman D., Newell D. G., Fullerton F., Yarnell J. W., Stacey A. R., Wald N., Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991 Jun 1;302(6788):1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Lew G. M., Klein P. D., Evans D. G., Evans D. J., Jr, Saeed Z. A., Malaty H. M. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992 May 1;116(9):705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeda S., Kanai F., Ogura K., Yoshida H., Ikenoue T., Takahashi M., Kawabe T., Shiratori Y., Omata M. High seropositivity of anti-CagA antibody in Helicobacter pylori-infected patients irrelevant to peptic ulcers and normal mucosa in Japan. Dig Dis Sci. 1997 Sep;42(9):1841–1847. doi: 10.1023/a:1018846723379. [DOI] [PubMed] [Google Scholar]
- Maeda S., Ogura K., Yoshida H., Kanai F., Ikenoue T., Kato N., Shiratori Y., Omata M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998 Mar;42(3):338–343. doi: 10.1136/gut.42.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehlke S., Kibler K., Kim J. G., Figura N., Small S. M., Graham D. Y., Go M. F. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996 Jul;91(7):1322–1325. [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Pan Z. J., van der Hulst R. W., Feller M., Xiao S. D., Tytgat G. N., Dankert J., van der Ende A. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients with peptic ulcer disease and those with chronic gastritis-associated dyspepsia. J Clin Microbiol. 1997 Jun;35(6):1344–1347. doi: 10.1128/jcm.35.6.1344-1347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Orentreich N., Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997 Mar;40(3):297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. A., Tummuru M. K., Miller G. G., Blaser M. J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995 May;63(5):1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama T., Fukuda S., Tanaka M., Mikami T., Saito Y., Munakata A. High prevalence of the CagA-positive Helicobacter pylori strains in Japanese asymptomatic patients and gastric cancer patients. Scand J Gastroenterol. 1997 May;32(5):465–468. doi: 10.3109/00365529709025082. [DOI] [PubMed] [Google Scholar]
- Sokolov B. P., Prockop D. J. A rapid and simple PCR-based method for isolation of cDNAs from differentially expressed genes. Nucleic Acids Res. 1994 Sep 25;22(19):4009–4015. doi: 10.1093/nar/22.19.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleischmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997 Aug 7;388(6642):539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Weel J. F., van der Hulst R. W., Gerrits Y., Roorda P., Feller M., Dankert J., Tytgat G. N., van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996 May;173(5):1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Bugnoli M., Ponzetto A., Morgando A., Figura N., Covacci A., Petracca R., Pennatini C., Censini S., Armellini D. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128 kilodalton protein (CagA) of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1993 Oct;12(10):739–745. doi: 10.1007/BF02098460. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]