Abstract
BACKGROUND—Helicobacter pylori, the main cause of chronic gastritis, is a class I gastric carcinogen. Chronic gastritis progresses to cancer through atrophy, metaplasia, and dysplasia. Precancerous phenotypic expression is generally associated with acquired genomic instability. AIM—To evaluate the effect of H pylori infection and its eradication on gastric histology, cell proliferation, DNA status, and oncogene expression. METHODS/SUBJECTS—Morphometric and immunohistochemical techniques were used to examine gastric mucosal biopsy specimens from eight controls, 10 patients with H pylori negative chronic gastritis, 53 with H pylori positive chronic gastritis, and 11 with gastric cancer. RESULTS—All patients with chronic gastritis were in a hyperproliferative state related to mucosal inflammation, regardless of H pylori infection. Atrophy was present in three of 10 patients with H pylori negative chronic gastritis and in 26 of 53 with H pylori positive chronic gastritis, associated in 18 with intestinal metaplasia. DNA content was abnormal in only 11 patients with atrophy and H pylori infection; eight of these also had c-Myc expression, associated in six cases with p53 expression. Fifty three patients with H pylori positive chronic gastritis were monitored for 12 months after antibiotic treatment: three dropped out; infection was eradicated in 45, in whom cell proliferation decreased in parallel with the reduction in gastritis activity; atrophy previously detected in 21/45 disappeared in five, regressed from moderate to mild in nine, and remained unchanged in seven; complete metaplasia disappeared in 4/14, and markers of genomic instability disappeared where previously present. In the five patients in whom H pylori persisted, atrophy, metaplasia, dysplasia, and markers of genomic instability remained unchanged. CONCLUSIONS—Chronic H pylori infection seems to be responsible for genomic instability in a subset of cases of H pylori positive chronic atrophic gastritis; eradication of H pylori infection can reverse inflammation and the related atrophy, metaplasia, and genomic instability. Keywords: H pylori infection; atrophic gastritis; genomic instability; eradication therapy
Full Text
The Full Text of this article is available as a PDF (400.8 KB).
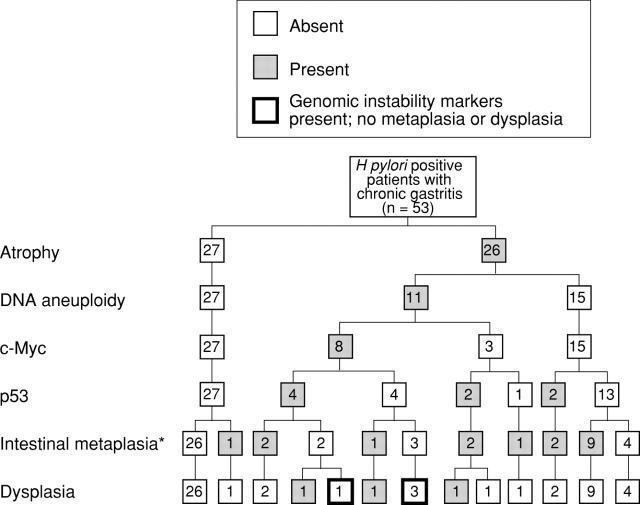
Figure 1 .
Distribution of histological and immunohistochemical features and DNA content in 53 H pylori positive patients with chronic gastritis. *16 complete and two incomplete.
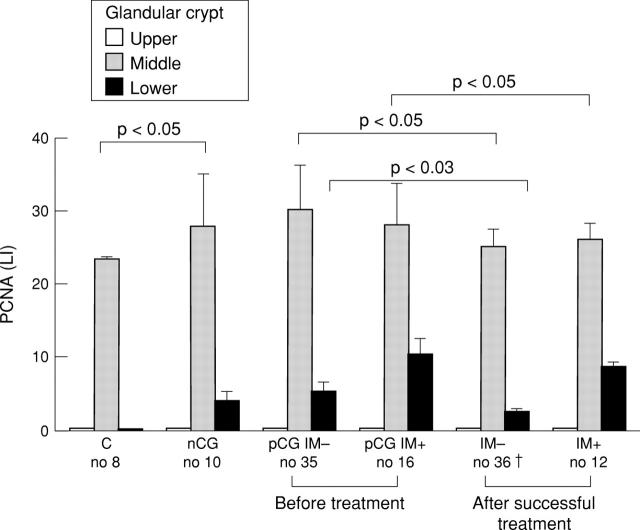
Figure 2 .
Immunohistochemical expression of proliferating cell nuclear antigen (PCNA) in gastric crypt sections (as indicated on the figure; a mean of five well oriented crypts for each patient) of patients with functional dyspepsia, H pylori negative chronic gastritis, or H pylori positive chronic gastritis, with or without complete intestinal metaplasia (IM), before and after H pylori eradication. LI, labelling index, expressed as percentage of PCNA positive nuclei; C, controls with functional dyspepsia; nCG, H pylori negative chronic gastritis; pCG IM−, H pylori positive chronic gastritis without intestinal metaplasia; pCG IM+, H pylori positive chronic gastritis with complete intestinal metaplasia; IM−, chronic gastritis without intestinal metaplasia after H pylori eradication; IM+, chronic gastritis with intestinal metaplasia after H pylori eradication. †Three patients dropped out; intestinal metaplasia disappeared in four subjects.
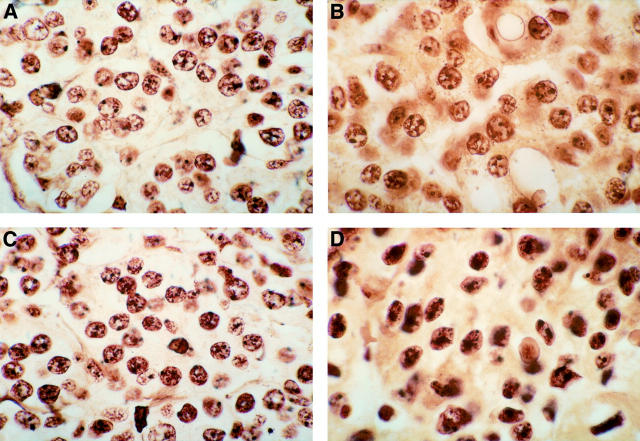
Figure 3 .
Proliferating cell nuclear antigen (PCNA) immunostaining of the gastric mucosa. (A) H pylori negative chronic gastritis with expression of PCNA in the nuclei of the cells of the glandular neck; (B) H pylori positive chronic gastritis; (C) H pylori positive chronic gastritis with intestinal metaplasia; (D) H pylori positive chronic gastritis after successful eradication treatment; (E) invasive gastric cancer showing high levels of PCNA positivity. Note the low labelling index of PCNA in the adjacent normal mucosa. Original magnifications: A, × 250; B, × 150; C × 400; D, × 250; E, × 150.
Figure 4 .
Silver stained nucleolar organiser regions (AgNORs) in gastric mucosa. (A) H pylori negative chronic gastritis: small to medium-sized AgNORs in the nuclei. (B) H pylori positive chronic gastritis: medium-sized irregular AgNORs in the nuclei. (C) H pylori positive chronic gastritis, after eradication treatment. (D) Gastric cancer: large, irregular and sometimes unusually shaped AgNORs in neoplastic cells. Original magnification: A, B, C and D, × 1000, oil immersion.
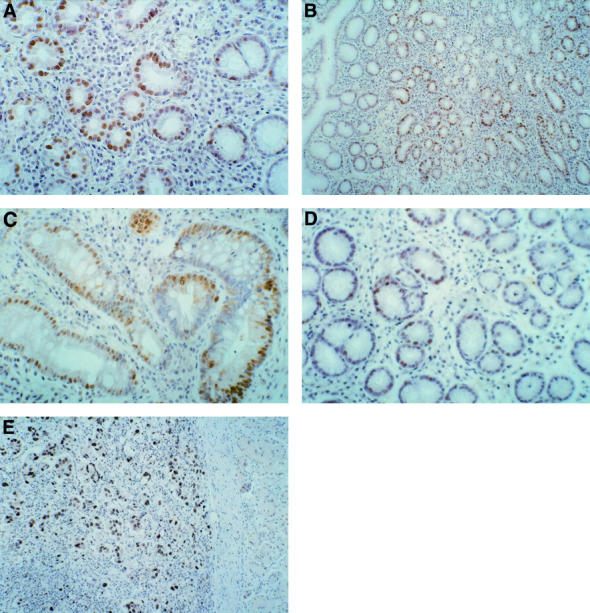
Figure 5 .
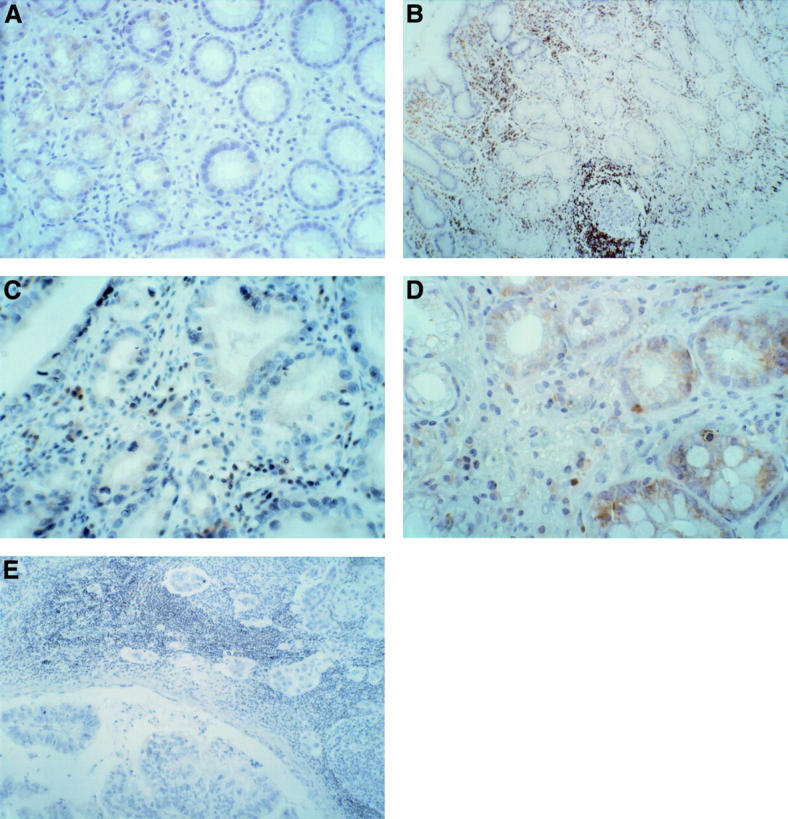
bcl-2 protein expression. (A) H pylori negative chronic gastritis: moderate bcl-2 expression. (B) H pylori positive chronic gastritis. (C) H pylori positive chronic gastritis with dysplasia: low bcl-2 expression. (D) H pylori positive chronic gastritis: moderate expression of bcl-2 protein in areas of intestinal metaplasia. (E) Nodal metastasis from gastric cancer: negative for bcl-2 protein in gastric cancer cells. Original magnifications: A, × 250; B, × 250; C, × 400; D, × 250; E, × 100.
Figure 6 .
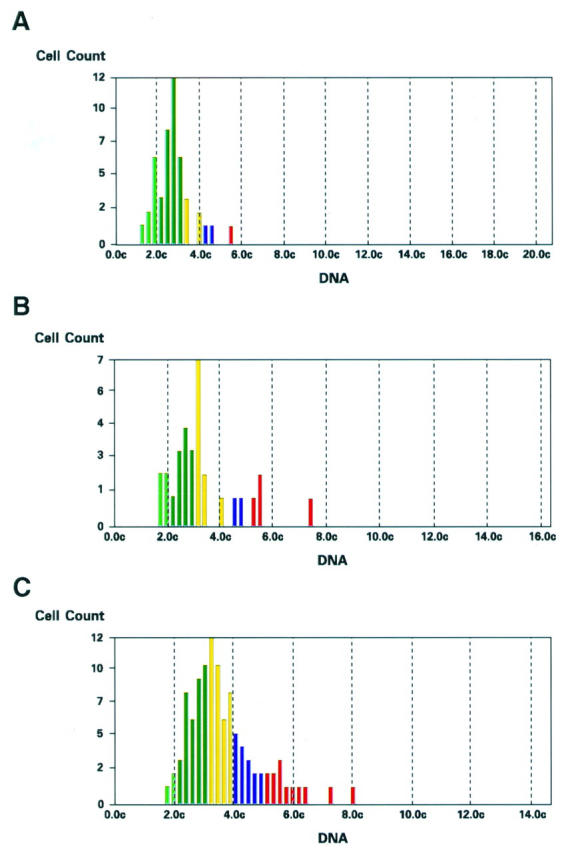
Representative DNA ploidy patterns in Feulgen stained sections of gastric mucosa. (A) H pylori positive chronic gastritis with moderate dysplasia: mild degree of aneuploidy. (B) H pylori positive chronic gastritis not responsive to eradication therapy without dysplasia: mild degree of aneuploidy. (C) Aneuploid pattern in gastric cancer (image analysis Quantimet 500C).
Figure 7 .
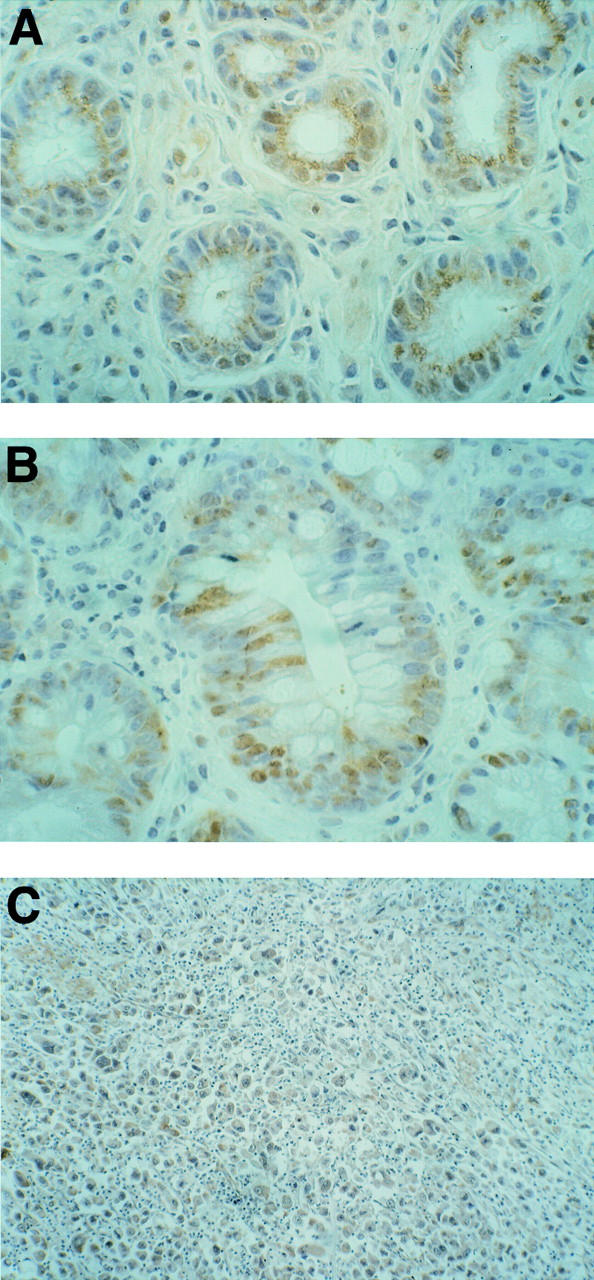
c-Myc expression. (A) H pylori positive chronic gastritis: low to moderate positivity for c-Myc expression. (B) H pylori positive chronic gastritis with intestinal metaplasia: moderate degree of positivity for c-Myc protein. (C) Gastric cancer: positivity for neoplastic cells for c-Myc protein. Original magnifications: A, × 400; B × 400; C × 100.
Figure 8 .
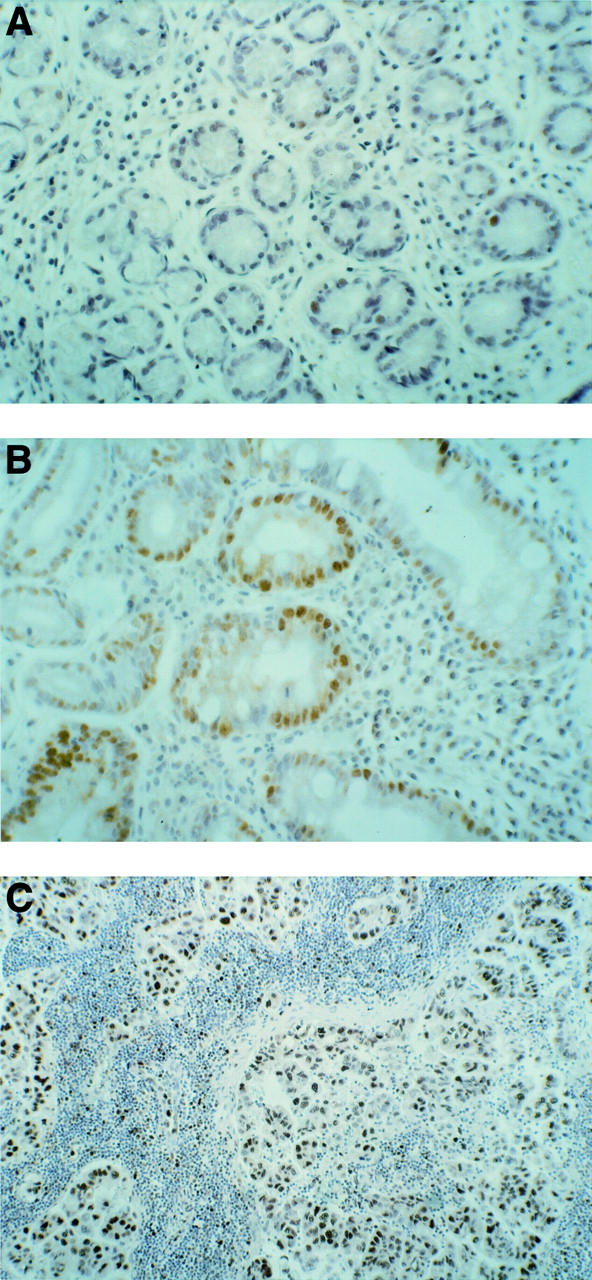
p53 protein expression. (A) H pylori positive chronic gastritis: sparse low positivity for p53 protein. (B) H pylori positive chronic gastritis with intestinal metaplasia: positivity for p53 protein in areas of intestinal metaplasia. (C) Gastric cancer: high positivity for p53 protein. Original magnifications: A, × 250; B, × 250; C, × 100.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Flemström G., Garner A., Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993 Oct;73(4):823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cancer: the rise of the genetic paradigm. Genes Dev. 1995 Jun 1;9(11):1309–1315. doi: 10.1101/gad.9.11.1309. [DOI] [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995 May 15;55(10):2111–2115. [PubMed] [Google Scholar]
- Brenes F., Ruiz B., Correa P., Hunter F., Rhamakrishnan T., Fontham E., Shi T. Y. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre- and post-eradication indices of proliferating cell nuclear antigen. Am J Gastroenterol. 1993 Nov;88(11):1870–1875. [PubMed] [Google Scholar]
- Bronner M. P., Culin C., Reed J. C., Furth E. E. The bcl-2 proto-oncogene and the gastrointestinal epithelial tumor progression model. Am J Pathol. 1995 Jan;146(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Burmer G. C., Rabinovitch P. S., Haggitt R. C., Crispin D. A., Brentnall T. A., Kolli V. R., Stevens A. C., Rubin C. E. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992 Nov;103(5):1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- Böcking A., Adler C. P., Common H. H., Hilgarth M., Granzen B., Auffermann W. Algorithm for a DNA-cytophotometric diagnosis and grading of malignancy. Anal Quant Cytol. 1984 Mar;6(1):1–8. [PubMed] [Google Scholar]
- Cahill R. J., Kilgallen C., Beattie S., Hamilton H., O'Morain C. Gastric epithelial cell kinetics in the progression from normal mucosa to gastric carcinoma. Gut. 1996 Feb;38(2):177–181. doi: 10.1136/gut.38.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo E., de la Calle-Martin O., Miquel R., Palacin A., Romero M., Fabregat V., Vives J., Cardesa A., Yague J. Loss of heterozygosity of p53 gene and p53 protein expression in human colorectal carcinomas. Cancer Res. 1991 Aug 15;51(16):4436–4442. [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Sordillo E. M., Ramey W. G., Reidy J., Holt P. R., Krajewski S., Reed J. C., Blaser M. J., Moss S. F. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem Biophys Res Commun. 1997 Oct 20;239(2):626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- Chow K. W., Bank S., Ahn J., Roberts J., Blumstein M., Kranz V. Helicobacter pylori infection does not increase gastric antrum mucosal cell proliferation. Am J Gastroenterol. 1995 Jan;90(1):64–66. [PubMed] [Google Scholar]
- Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 (Suppl 1):S37–S43. [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735–6740. [PubMed] [Google Scholar]
- Correa P., Miller M. J. Helicobacter pylori and gastric atrophy--cancer paradoxes. J Natl Cancer Inst. 1995 Dec 6;87(23):1731–1732. doi: 10.1093/jnci/87.23.1731. [DOI] [PubMed] [Google Scholar]
- Correa P., Ruiz B., Shi T. Y., Janney A., Sobhan M., Torrado J., Hunter F. Helicobacter pylori and nucleolar organizer regions in the gastric antral mucosa. Am J Clin Pathol. 1994 May;101(5):656–660. doi: 10.1093/ajcp/101.5.656. [DOI] [PubMed] [Google Scholar]
- Craanen M. E., Blok P., Dekker W., Offerhaus G. J., Tytgat G. N. Chronology of p53 protein accumulation in gastric carcinogenesis. Gut. 1995 Jun;36(6):848–852. doi: 10.1136/gut.36.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker J., Boldy D. A., Egan M. J. How should we count AgNORS? Proposals for a standardized approach. J Pathol. 1989 Jul;158(3):185–188. doi: 10.1002/path.1711580303. [DOI] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Egan M., Freeth M., Crocker J. Intraepithelial neoplasia, human papilloma virus infection and argyrophilic nucleoprotein in cervical epithelium. Histopathology. 1988 Nov;13(5):561–567. doi: 10.1111/j.1365-2559.1988.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Enoch T., Norbury C. Cellular responses to DNA damage: cell-cycle checkpoints, apoptosis and the roles of p53 and ATM. Trends Biochem Sci. 1995 Oct;20(10):426–430. doi: 10.1016/s0968-0004(00)89093-3. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Flyger H. L., Christensen I. J., Thorup J., Håkansson T. U., Nørgaard T. DNA aneuploidy in gastric carcinoma. Flow cytometric data related to survival, location, and histopathologic findings. Scand J Gastroenterol. 1995 Mar;30(3):258–264. doi: 10.3109/00365529509093274. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Li X., Cahill R. J., Andrutis K., Rustgi A. K., Odze R., Wang T. C. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996 Jan;110(1):155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- Fraser A. G., Sim R., Sankey E. A., Dhillon A. P., Pounder R. E. Effect of eradication of Helicobacter pylori on gastric epithelial cell proliferation. Aliment Pharmacol Ther. 1994 Apr;8(2):167–173. doi: 10.1111/j.1365-2036.1994.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Genta R. M. Helicobacter pylori, inflammation, mucosal damage, and apoptosis: pathogenesis and definition of gastric atrophy. Gastroenterology. 1997 Dec;113(6 Suppl):S51–S55. doi: 10.1016/s0016-5085(97)80012-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. P., Kumar D., Sánchez R. L. AgNOR area measurements differentiate benign and malignant melanocytic lesions more accurately than simple counting. Am J Dermatopathol. 1994 Aug;16(4):372–376. doi: 10.1097/00000372-199408000-00003. [DOI] [PubMed] [Google Scholar]
- Henry M. A., Tange J. D. Lesions of the renal papilla induced by paracetamol. J Pathol. 1987 Jan;151(1):11–19. doi: 10.1002/path.1711510103. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hurlimann J., Saraga E. P. Expression of p53 protein in gastric carcinomas. Association with histologic type and prognosis. Am J Surg Pathol. 1994 Dec;18(12):1247–1253. doi: 10.1097/00000478-199412000-00008. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Monden T., Morimoto H., Murotani M., Miyoshi Y., Kobayashi T., Shimano T., Mori T. Immunohistochemical study of p53 expression in microwave-fixed, paraffin-embedded sections of colorectal carcinoma and adenoma. Am J Clin Pathol. 1992 Feb;97(2):244–249. doi: 10.1093/ajcp/97.2.244. [DOI] [PubMed] [Google Scholar]
- Kitayama Y., Nakamura S., Sugimura H., Kino I. Cytophotometric and flow cytometric DNA content of isolated glands in gastric neoplasia. Gut. 1995 Apr;36(4):516–521. doi: 10.1136/gut.36.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lauwers G. Y., Scott G. V., Karpeh M. S. Immunohistochemical evaluation of bcl-2 protein expression in gastric adenocarcinomas. Cancer. 1995 May 1;75(9):2209–2213. doi: 10.1002/1097-0142(19950501)75:9<2209::aid-cncr2820750904>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Levine D. S. Barrett's oesophagus and p53. Lancet. 1994 Jul 23;344(8917):212–213. doi: 10.1016/s0140-6736(94)92993-9. [DOI] [PubMed] [Google Scholar]
- Levine D. S., Rabinovitch P. S., Haggitt R. C., Blount P. L., Dean P. J., Rubin C. E., Reid B. J. Distribution of aneuploid cell populations in ulcerative colitis with dysplasia or cancer. Gastroenterology. 1991 Nov;101(5):1198–1210. doi: 10.1016/0016-5085(91)90068-v. [DOI] [PubMed] [Google Scholar]
- Lynch D. A., Mapstone N. P., Clarke A. M., Jackson P., Dixon M. F., Quirke P., Axon A. T. Cell proliferation in the gastric corpus in Helicobacter pylori associated gastritis and after gastric resection. Gut. 1995 Mar;36(3):351–353. doi: 10.1136/gut.36.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. A., Mapstone N. P., Clarke A. M., Sobala G. M., Jackson P., Morrison L., Dixon M. F., Quirke P., Axon A. T. Cell proliferation in Helicobacter pylori associated gastritis and the effect of eradication therapy. Gut. 1995 Mar;36(3):346–350. doi: 10.1136/gut.36.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero J. M., de Caestecker J. S., Corbishley C. M., McCormick C., Northfield T. C. Gastric DNA content in postgastrectomy patients. Relationship to mucosal dysplasia. Cancer. 1996 Jan 1;77(1):19–24. doi: 10.1002/(SICI)1097-0142(19960101)77:1<19::AID-CNCR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Mauch F., Bode G., Ditschuneit H., Malfertheiner P. Demonstration of a phospholipid-rich zone in the human gastric epithelium damaged by Helicobacter pylori. Gastroenterology. 1993 Dec;105(6):1698–1704. doi: 10.1016/0016-5085(93)91065-p. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Calam J., Agarwal B., Wang S., Holt P. R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996 Apr;38(4):498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. W., Wielenga V. J., Polak M. M., van den Berg F. M., Adolf G. R., Herrlich P., Pals S. T., Offerhaus G. J. Expression of mutant p53 protein and CD44 variant proteins in colorectal tumorigenesis. Gut. 1995 Jan;36(1):76–80. doi: 10.1136/gut.36.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Asagoe K., Dekigai H., Kusaka S., Saita H., Kita T. Products of neutrophil metabolism increase ammonia-induced gastric mucosal damage. Dig Dis Sci. 1995 Feb;40(2):268–273. doi: 10.1007/BF02065408. [DOI] [PubMed] [Google Scholar]
- Mégraud F., Neman-Simha V., Brügmann D. Further evidence of the toxic effect of ammonia produced by Helicobacter pylori urease on human epithelial cells. Infect Immun. 1992 May;60(5):1858–1863. doi: 10.1128/iai.60.5.1858-1863.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone G., d'Armiento F., Corso G., Coscione P., Esposito M., Budillon G. Lipids of human gastric mucosa: effect of Helicobacter pylori infection and nonalcoholic cirrhosis. Gastroenterology. 1994 Aug;107(2):362–368. doi: 10.1016/0016-5085(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Ohyama S., Yonemura Y., Miyazaki I. Prognostic value of S-phase fraction and DNA ploidy studied with in vivo administration of bromodeoxyuridine on human gastric cancers. Cancer. 1990 Jan 1;65(1):116–121. doi: 10.1002/1097-0142(19900101)65:1<116::aid-cncr2820650124>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Panella C., Ierardi E., Polimeno L., Balzano T., Ingrosso M., Amoruso A., Traversa A., Francavilla A. Proliferative activity of gastric epithelium in progressive stages of Helicobacter pylori infection. Dig Dis Sci. 1996 Jun;41(6):1132–1138. doi: 10.1007/BF02088228. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Orentreich N., Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997 Mar;40(3):297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. J., Haggitt R. C., Rubin C. E., Roth G., Surawicz C. M., Van Belle G., Lewin K., Weinstein W. M., Antonioli D. A., Goldman H. Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol. 1988 Feb;19(2):166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- Rugge M., Cassaro M., Leandro G., Baffa R., Avellini C., Bufo P., Stracca V., Battaglia G., Fabiano A., Guerini A. Helicobacter pylori in promotion of gastric carcinogenesis. Dig Dis Sci. 1996 May;41(5):950–955. doi: 10.1007/BF02091536. [DOI] [PubMed] [Google Scholar]
- Sozzi M., Valentini M., Figura N., De Paoli P., Tedeschi R. M., Gloghini A., Serraino D., Poletti M., Carbone A. Atrophic gastritis and intestinal metaplasia in Helicobacter pylori infection: the role of CagA status. Am J Gastroenterol. 1998 Mar;93(3):375–379. doi: 10.1111/j.1572-0241.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Starzynska T., Bromley M., Ghosh A., Stern P. L. Prognostic significance of p53 overexpression in gastric and colorectal carcinoma. Br J Cancer. 1992 Sep;66(3):558–562. doi: 10.1038/bjc.1992.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara H., Hattori T., Fujita S., Hirose K., Fukuda M. Regional ploidy variations in signet ring cell carcinomas of the stomach. Cancer. 1990 Jan 1;65(1):122–129. doi: 10.1002/1097-0142(19900101)65:1<122::aid-cncr2820650125>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tatsuta M., Iishi H., Baba M., Nakaizumi A., Uehara H., Taniguchi H. Expression of c-myc mRNA as an aid in histologic differentiation of adenoma from well differentiated adenocarcinoma in the stomach. Cancer. 1994 Apr 1;73(7):1795–1799. doi: 10.1002/1097-0142(19940401)73:7<1795::aid-cncr2820730704>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Tsujii M., Kawano S., Tsuji S., Nagano K., Ito T., Hayashi N., Fusamoto H., Kamada T., Tamura K. Ammonia: a possible promotor in Helicobacter pylori-related gastric carcinogenesis. Cancer Lett. 1992 Jul 31;65(1):15–18. doi: 10.1016/0304-3835(92)90207-c. [DOI] [PubMed] [Google Scholar]
- Van der Valk P., Meijer C. J. The non-Hodgkin's lymphomas: old and new thinking. Histopathology. 1988 Oct;13(4):367–384. doi: 10.1111/j.1365-2559.1988.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Yabuki N., Sasano H., Tobita M., Imatani A., Hoshi T., Kato K., Ohara S., Asaki S., Toyota T., Nagura H. Analysis of cell damage and proliferation in Helicobacter pylori-infected human gastric mucosa from patients with gastric adenocarcinoma. Am J Pathol. 1997 Sep;151(3):821–829. [PMC free article] [PubMed] [Google Scholar]
- Younes M., Lebovitz R. M., Lechago L. V., Lechago J. p53 protein accumulation in Barrett's metaplasia, dysplasia, and carcinoma: a follow-up study. Gastroenterology. 1993 Dec;105(6):1637–1642. doi: 10.1016/0016-5085(93)91058-p. [DOI] [PubMed] [Google Scholar]
- van der Hulst R. W., van der Ende A., Dekker F. W., Ten Kate F. J., Weel J. F., Keller J. J., Kruizinga S. P., Dankert J., Tytgat G. N. Effect of Helicobacter pylori eradication on gastritis in relation to cagA: a prospective 1-year follow-up study. Gastroenterology. 1997 Jul;113(1):25–30. doi: 10.1016/s0016-5085(97)70076-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.