Abstract
BACKGROUND—Mitogen activated protein kinases (MAPKs) play a central role in the regulation of both cell growth and differentiation. They are involved in signal transduction of oncogenes and growth factors. The role of MAPK in colonic carcinoma is unknown. AIMS—To establish whether the expression and activity of p42/44 MAPKs are altered in colorectal tumours as compared with normal mucosa. METHODS—The expression and activity of p42/p44 MAPK were investigated in 22 colorectal carcinomas, four adenomas, and the corresponding normal colorectal mucosa by the use of western blotting, immunoprecipitation, and in vitro kinase assays. RESULTS—After immunoprecipitation with an antibody specific for p42 MAPK, we found significant inactivation of p42 MAPK in colonic carcinomas as well as in adenomas, whereas most sample pairs showed only minor differences in p42 MAPK expression. Investigation of MAPK with an antibody capable of detecting both p42 and p44 MAPK showed a slight but significant decrease in p44 MAPK content in malignant tissues. With this antibody, only minor alterations in MAPK activity and no correlation with p42 MAPK activity were found. CONCLUSIONS—Inactivation of p42 MAPK could be associated with colonic carcinogenesis. Keywords: mitogen activated protein kinase (MAPK); Raf-1; colorectal cancer
Full Text
The Full Text of this article is available as a PDF (129.9 KB).
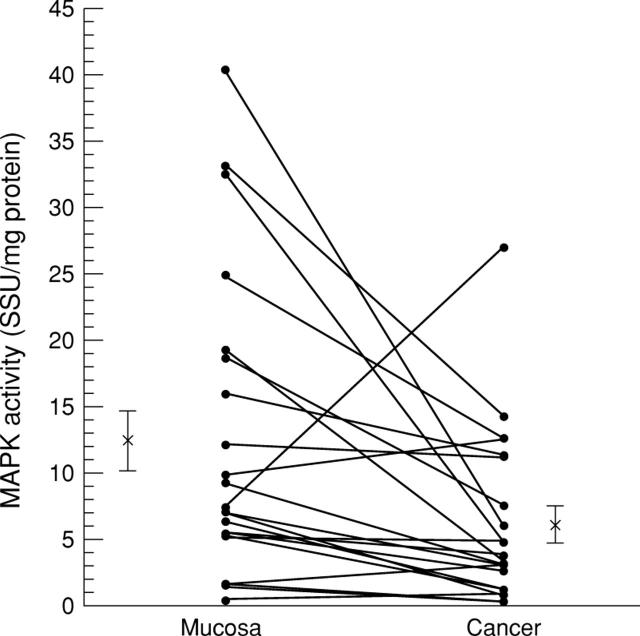
Figure 1 .
Activity of p42 mitogen activated protein kinase (MAPK) in colorectal cancer. The activities (seastar units (SSU)/mg protein) of corresponding colonic cancer/mucosa samples (n = 22) are connected by lines. Asterisks with error bars indicate the mean values and standard errors. Activities were examined by in vitro kinase assay after immunoprecipitation with sc-154.
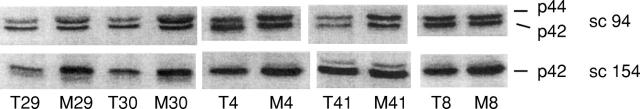
Figure 2 .
Anti-mitogen activated protein kinase (MAPK) western blots of five representative paired samples of colon cancer (T) and mucosa (M). For the immunoblots shown in the upper row the sc-94 antibody, which detects p42 MAPK and p44 MAPK, was used. The blots shown below were obtained with p42 MAPK-specific sc-154.
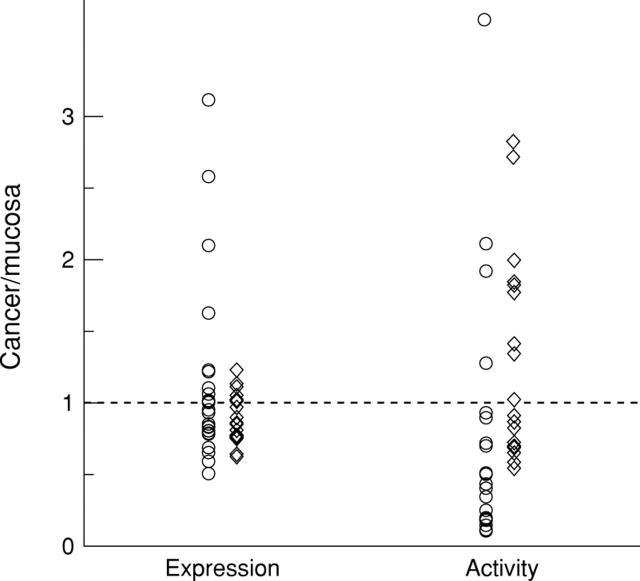
Figure 3 .
Relative activity and expression of p42 mitogen activated protein kinase (MAPK) and p44/42 MAPK. The cancer/mucosa ratio of every sample pair is indicated. p42 MAPK was assayed by the use of sc-154 and is indicated by circles. p44/42 MAPK was determined using sc-94 and is displayed by diamonds.
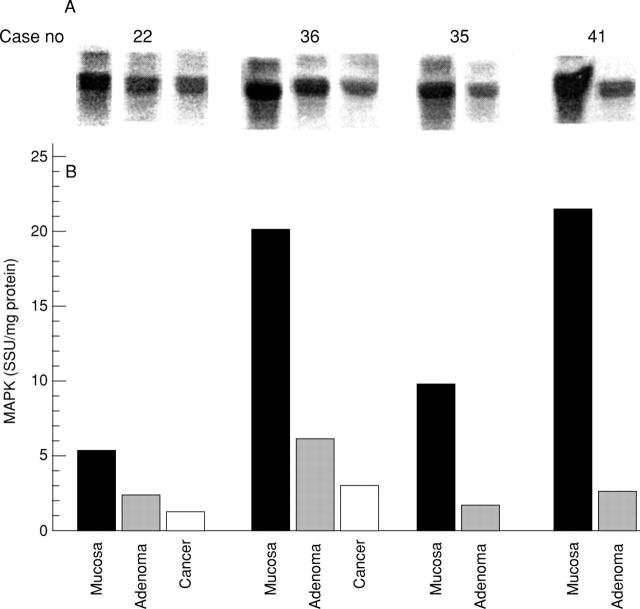
Figure 4 .
Activity of p42 mitogen activated protein kinase (MAPK) in colonic adenomas. p42 MAPK activity was assayed in four colonic adenomas, two of them presenting together with carcinomas. (A) Autoradiographs of phosphorylated myelin basic protein; (B) bar graph of the activity (seastar units (SSU)/mg protein) quantified with a phosphoimager.
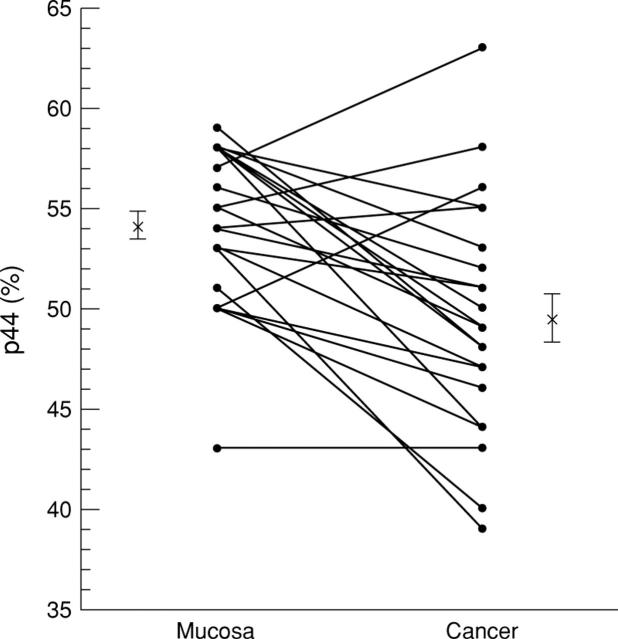
Figure 5 .
Relative expression of p44 mitogen activated protein kinase (MAPK) in colonic cancer. Immunoblots using sc-94 were quantified densitometrically. Points indicate the proportion of p44 MAPK ((p44 × 100)/(p44+p42)). The values (percentage) of corresponding colonic cancer/mucosa samples (n = 22) are connected by lines. Asterisks with error bars indicate the mean values and standard errors.
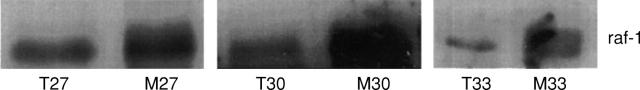
Figure 6 .
Raf-1 mobility shifts of three paired samples of colon cancer (T) and mucosa (M).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Li P., Marsden L. A., Williams N., Roberts T. M., Sturgill T. W. Raf-1 is a potential substrate for mitogen-activated protein kinase in vivo. Biochem J. 1991 Jul 15;277(Pt 2):573–576. doi: 10.1042/bj2770573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhattar J., Losi L., Chaubert P., Givel J. C., Costa J. Prognostic significance of K-ras mutations in colorectal carcinoma. Gastroenterology. 1993 Apr;104(4):1044–1048. doi: 10.1016/0016-5085(93)90272-e. [DOI] [PubMed] [Google Scholar]
- Blagosklonny M. V., Schulte T., Nguyen P., Trepel J., Neckers L. M. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway. Cancer Res. 1996 Apr 15;56(8):1851–1854. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cano E., Mahadevan L. C. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995 Mar;20(3):117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Chajry N., Martin P. M., Cochet C., Berthois Y. Regulation of p42 mitogen-activated-protein kinase activity by protein phosphatase 2A under conditions of growth inhibition by epidermal growth factor in A431 cells. Eur J Biochem. 1996 Jan 15;235(1-2):97–102. doi: 10.1111/j.1432-1033.1996.00097.x. [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P., Marshall C. J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994 Jun 17;77(6):841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Eggstein S., Manthey G., Hirsch T., Baas F., Specht B. U., Farthmann E. H. Raf-1 kinase, epidermal growth factor receptors, and mutant Ras proteins in colonic carcinomas. Dig Dis Sci. 1996 Jun;41(6):1069–1075. doi: 10.1007/BF02088221. [DOI] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Guillem J. G., O'Brian C. A., Fitzer C. J., Forde K. A., LoGerfo P., Treat M., Weinstein I. B. Altered levels of protein kinase C and Ca2+-dependent protein kinases in human colon carcinomas. Cancer Res. 1987 Apr 15;47(8):2036–2039. [PubMed] [Google Scholar]
- Häfner S., Adler H. S., Mischak H., Janosch P., Heidecker G., Wolfman A., Pippig S., Lohse M., Ueffing M., Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994 Oct;14(10):6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp R., Noelke B., Sauter G., Schildberg F. W., Paumgartner G., Pfeiffer A. Altered protein kinase C activity in biopsies of human colonic adenomas and carcinomas. Cancer Res. 1991 Jan 1;51(1):205–210. [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. M., Cobb M. H., Blackshear P. J. Evidence that extracellular signal-regulated kinases are the insulin-activated Raf-1 kinase kinases. J Biol Chem. 1992 Jan 15;267(2):1088–1092. [PubMed] [Google Scholar]
- Mamajiwalla S. N., Burgess D. R. Differential regulation of the activity of the 42 kD mitogen activated protein kinase (p42mapk) during enterocyte differentiation in vivo. Oncogene. 1995 Jul 20;11(2):377–386. [PubMed] [Google Scholar]
- Nebreda A. R. Inactivation of MAP kinases. Trends Biochem Sci. 1994 Jan;19(1):1–2. doi: 10.1016/0968-0004(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Nishida E., Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993 Apr;18(4):128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- Oka H., Chatani Y., Hoshino R., Ogawa O., Kakehi Y., Terachi T., Okada Y., Kawaichi M., Kohno M., Yoshida O. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995 Sep 15;55(18):4182–4187. [PubMed] [Google Scholar]
- Porras A., Muszynski K., Rapp U. R., Santos E. Dissociation between activation of Raf-1 kinase and the 42-kDa mitogen-activated protein kinase/90-kDa S6 kinase (MAPK/RSK) cascade in the insulin/Ras pathway of adipocytic differentiation of 3T3 L1 cells. J Biol Chem. 1994 Apr 29;269(17):12741–12748. [PubMed] [Google Scholar]
- Troppmair J., Bruder J. T., App H., Cai H., Liptak L., Szeberényi J., Cooper G. M., Rapp U. R. Ras controls coupling of growth factor receptors and protein kinase C in the membrane to Raf-1 and B-Raf protein serine kinases in the cytosol. Oncogene. 1992 Sep;7(9):1867–1873. [PubMed] [Google Scholar]
- Ward Y., Gupta S., Jensen P., Wartmann M., Davis R. J., Kelly K. Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature. 1994 Feb 17;367(6464):651–654. doi: 10.1038/367651a0. [DOI] [PubMed] [Google Scholar]
- Watabe M., Masuda Y., Nakajo S., Yoshida T., Kuroiwa Y., Nakaya K. The cooperative interaction of two different signaling pathways in response to bufalin induces apoptosis in human leukemia U937 cells. J Biol Chem. 1996 Jun 14;271(24):14067–14072. doi: 10.1074/jbc.271.24.14067. [DOI] [PubMed] [Google Scholar]