Abstract
BACKGROUND—High mobility group (HMG) non-histone chromosomal proteins HMG1 and HMG2 have been identified as novel antigens of perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCAs), and the existence of anti-HMG1 and anti-HMG2 antibodies in a population of patients with ulcerative colitis has been reported. AIMS—To investigate whether HMG1 and HMG2 are target antigens for p-ANCAs in autoimmune hepatitis (AIH). PATIENTS—Serum samples from 28 patients with AIH, 44 patients with primary biliary cirrhosis (PBC), 27 patients with chronic hepatitis C, and 23 patients with chronic hepatitis B were tested. METHODS—ANCAs were detected by routine indirect immunofluorescence (IIF). Anti-HMG1 and anti-HMG2 antibodies were assayed by enzyme linked immunosorbent assay. RESULTS—p-ANCAs were detected in 89% (25/28) of patients with AIH, 36% (16/44) of patients with PBC, 11% (3/27) of patients with chronic hepatitis C, and 13% (3/23) of patients with chronic hepatitis B. Anti-HMG1 and/or anti-HMG2 antibodies were detected in 89% (25/28) of patients with AIH, 70% (31/44) with PBC, 26% (7/27) with chronic hepatitis C, and 9% (2/23) with chronic hepatitis B. In AIH, anti-HMG1 and/or anti-HMG2 antibodies were detected in 96% (24/25) of p-ANCA positive patients. The p-ANCA staining pattern detected by IIF using sera from patients with AIH disappeared or decreased in titre after preincubation with a mixture of HMG1/HMG2. The presence and titres of those antibodies in AIH correlated significantly with those of p-ANCA, but not with those of anti-nuclear antibody or anti-smooth muscle antibody. CONCLUSIONS—HMG1 and HMG2 are significant target antigens of p-ANCA in AIH. Keywords: perinuclear anti-neutrophil cytoplasmic antibodies; chromosomal proteins; high mobility group 1 and 2; autoimmune; hepatitis
Full Text
The Full Text of this article is available as a PDF (180.5 KB).
Figure 1 .
Perinuclear anti-neutrophil cytoplasmic antibody activities on indirect immunofluorescence. Sera from patients with autoimmune hepatitis (A, B) and primary biliary cirrhosis (C, D), and an affinity purified anti-HMG1/HMG2 antibody (E, F) showing perinuclear staining on ethanol fixed neutrophils (A, C, E) and cytoplasmic or negative staining on formalin fixed neutrophils (B, D, F).
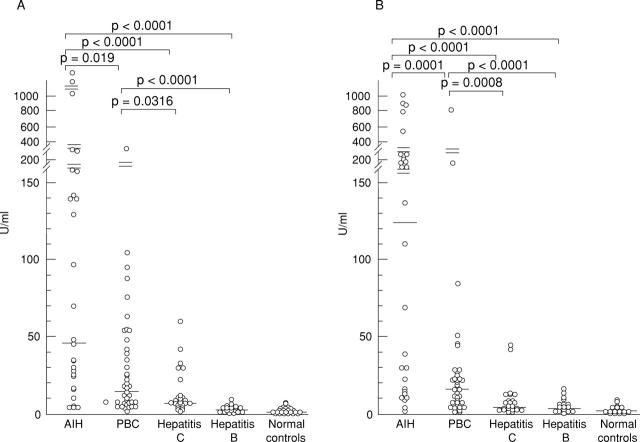
Figure 2 .
Titres of antibodies to HMG1 (A) and HMG2 (B) determined by ELISA. AIH, autoimmune hepatitis; PBC, primary biliary cirrhosis. The solid lines represent median values of antibody titres. p values by Mann-Whitney U test.
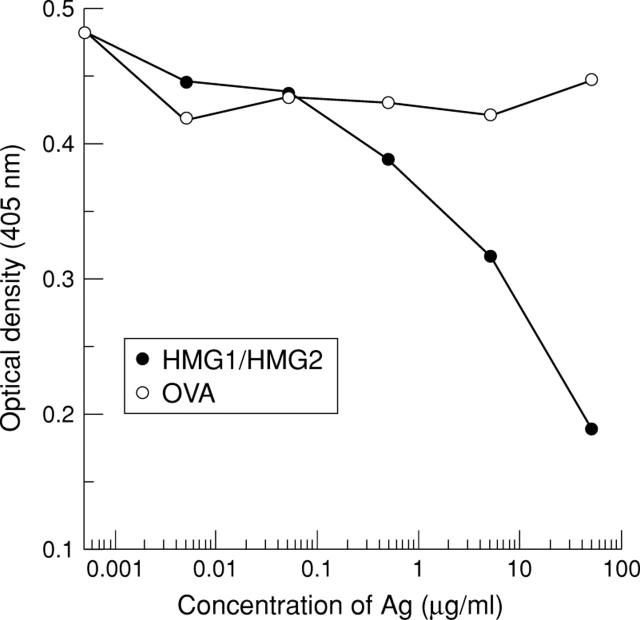
Figure 3 .
Competitive inhibition ELISA. Anti-HMG1/HMG2 antibody positive serum from a patient with autoimmune hepatitis was preincubated with a mixture of HMG1 and HMG2 or ovalbumin (OVA) at the concentrations indicated. In a subsequent HMG1/HMG2 ELISA, the reactivity decreased in a dose dependent manner when preincubated with HMG1/HMG2, but not with ovalbumin.
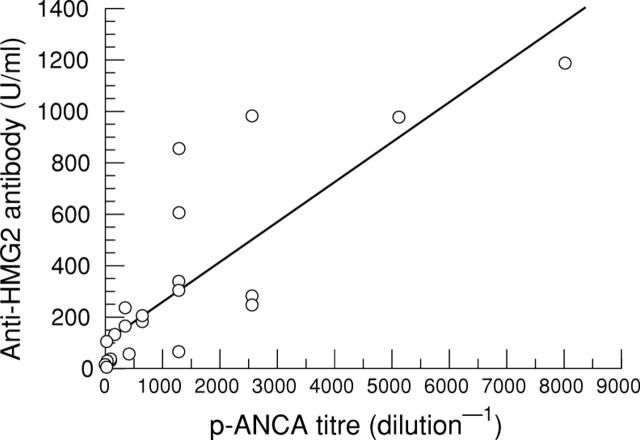
Figure 4 .
Correlation between the titres of anti-HMG2 antibody and perinuclear anti-neutrophil cytoplasmic antibodies in autoimmune hepatitis. A positive correlation was found between the two parameters (r = 0.8264, p<0.0001, Spearman rank correlation test).
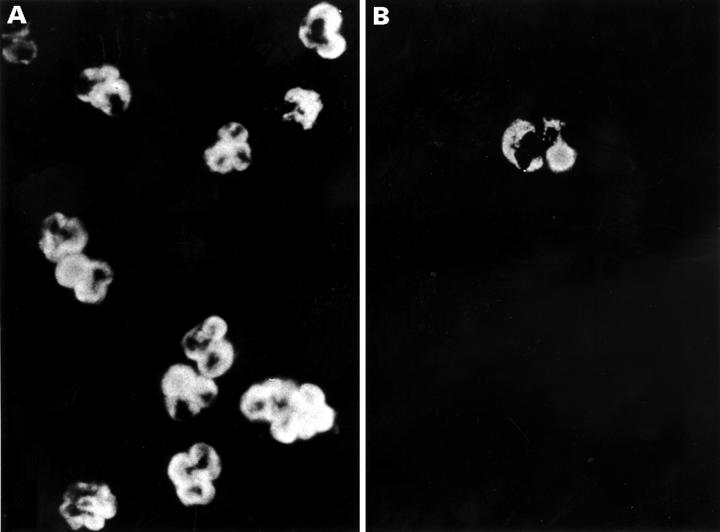
Figure 5 .
Disappearance of perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) staining after preincubation with specific antigens. Serum from a patient with autoimmune hepatitis with a p-ANCA titre of 1280 was diluted at 1:160 and preincubated with an equal volume of 100 µg/ml of a mixture of HMG1 and HMG2 (B) or phosphate buffered saline (A). Subsequent IIF was performed by each solution, and photographs were taken at the same exposure. No fluorescence was visible in (B), where the only bright cell is an eosinophil with spontaneous fluorescence.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batoni G., Esin S., Harris R. A., Källenius G., Svenson S. B., Andersson R., Campa M., Wigzell H. Gammadelta+ and CD4+ alphabeta+ human T cell subset responses upon stimulation with various Mycobacterium tuberculosis soluble extracts. Clin Exp Immunol. 1998 Apr;112(1):52–62. doi: 10.1046/j.1365-2249.1998.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Lehn D. A., Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990 Jul 30;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Bustin M., Neihart N. K. Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells. Cell. 1979 Jan;16(1):181–189. doi: 10.1016/0092-8674(79)90199-5. [DOI] [PubMed] [Google Scholar]
- Cambridge G., Rampton D. S., Stevens T. R., McCarthy D. A., Kamm M., Leaker B. Anti-neutrophil antibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1992 May;33(5):668–674. doi: 10.1136/gut.33.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr R. H., Targan S. R., Landers C. J., Sutherland L. R., Shanahan F. Anti-neutrophil cytoplasmic antibodies in ulcerative colitis. Comparison with other colitides/diarrheal illnesses. Gastroenterology. 1991 Jun;100(6):1590–1596. doi: 10.1016/0016-5085(91)90657-7. [DOI] [PubMed] [Google Scholar]
- Einck L., Bustin M. The intracellular distribution and function of the high mobility group chromosomal proteins. Exp Cell Res. 1985 Feb;156(2):295–310. doi: 10.1016/0014-4827(85)90539-7. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988 Jun 23;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Halbwachs-Mecarelli L., Nusbaum P., Noël L. H., Reumaux D., Erlinger S., Grünfeld J. P., Lesavre P. Antineutrophil cytoplasmic antibodies (ANCA) directed against cathepsin G in ulcerative colitis, Crohn's disease and primary sclerosing cholangitis. Clin Exp Immunol. 1992 Oct;90(1):79–84. doi: 10.1111/j.1365-2249.1992.tb05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardarson S., Labrecque D. R., Mitros F. A., Neil G. A., Goeken J. A. Antineutrophil cytoplasmic antibody in inflammatory bowel and hepatobiliary diseases. High prevalence in ulcerative colitis, primary sclerosing cholangitis, and autoimmune hepatitis. Am J Clin Pathol. 1993 Mar;99(3):277–281. doi: 10.1093/ajcp/99.3.277. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., McFarlane I. G. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993 Oct;18(4):998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- Klein R., Eisenburg J., Weber P., Seibold F., Berg P. A. Significance and specificity of antibodies to neutrophils detected by western blotting for the serological diagnosis of primary sclerosing cholangitis. Hepatology. 1991 Dec;14(6):1147–1152. [PubMed] [Google Scholar]
- Lesavre P., Nusbaum P., Halbwachs-Mecarelli L. Methods of detection of anti-cathepsin G autoantibodies in human. Adv Exp Med Biol. 1993;336:257–261. [PubMed] [Google Scholar]
- Lüdemann J., Utecht B., Gross W. L. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990 Jan 1;171(1):357–362. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Brown D., Kerby S., Rudzinski I., Polte T., Randhawa Z., Seidman M. M. Sequence of human HMG2 cDNA. Nucleic Acids Res. 1991 Dec 11;19(23):6643–6643. doi: 10.1093/nar/19.23.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Sparatore B., Patrone M., Pessino A., Passalacqua M., Pontremoli S. Extracellular release of the 'differentiation enhancing factor', a HMG1 protein type, is an early step in murine erythroleukemia cell differentiation. FEBS Lett. 1995 Jul 24;368(3):466–470. doi: 10.1016/0014-5793(95)00716-m. [DOI] [PubMed] [Google Scholar]
- Merenmies J., Pihlaskari R., Laitinen J., Wartiovaara J., Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991 Sep 5;266(25):16722–16729. [PubMed] [Google Scholar]
- Mulder A. H., Horst G., Haagsma E. B., Limburg P. C., Kleibeuker J. H., Kallenberg C. G. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993 Mar;17(3):411–417. [PubMed] [Google Scholar]
- Nölle B., Specks U., Lüdemann J., Rohrbach M. S., DeRemee R. A., Gross W. L. Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener granulomatosis. Ann Intern Med. 1989 Jul 1;111(1):28–40. doi: 10.7326/0003-4819-111-1-28. [DOI] [PubMed] [Google Scholar]
- Ohta Y. Report of the research subgroup of autoimmune hepatitis/primary biliary cirrhosis. Gastroenterol Jpn. 1993 Mar;28 (Suppl 4):128–133. doi: 10.1007/BF02782905. [DOI] [PubMed] [Google Scholar]
- Orth T., Gerken G., Kellner R., Meyer zum Büschenfelde K. H., Mayet W. J. Actin is a target antigen of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune hepatitis type-1. J Hepatol. 1997 Jan;26(1):37–47. doi: 10.1016/s0168-8278(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Raulo E., Merenmies J., Nolo R., Kajander E. O., Baumann M., Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993 Sep 15;268(26):19726–19738. [PubMed] [Google Scholar]
- Peen E., Almer S., Bodemar G., Rydén B. O., Sjölin C., Tejle K., Skogh T. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993 Jan;34(1):56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Shanahan F., Landers C., Ganz T., Targan S. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990 Aug;86(2):202–210. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- Seibold F., Weber P., Klein R., Berg P. A., Wiedmann K. H. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992 May;33(5):657–662. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H., Tsuda K., Yoshida M. Primary structure of non-histone chromosomal protein HMG2 revealed by the nucleotide sequence. Biochemistry. 1990 May 8;29(18):4419–4423. doi: 10.1021/bi00470a022. [DOI] [PubMed] [Google Scholar]
- Shirakawa H., Yoshida M. Structure of a gene coding for human HMG2 protein. J Biol Chem. 1992 Apr 5;267(10):6641–6645. [PubMed] [Google Scholar]
- Sobajima J., Ozaki S., Okazaki T., Osakada F., Sumita S., Mori K., Nakao K. Anti-neutrophil cytoplasmic antibodies (ANCA) in ulcerative colitis: anti-cathepsin G and a novel antibody correlate with a refractory type. Clin Exp Immunol. 1996 Jul;105(1):120–124. doi: 10.1046/j.1365-2249.1996.d01-724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobajima J., Ozaki S., Osakada F., Uesugi H., Shirakawa H., Yoshida M., Nakao K. Novel autoantigens of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) in ulcerative colitis: non-histone chromosomal proteins, HMG1 and HMG2. Clin Exp Immunol. 1997 Jan;107(1):135–140. doi: 10.1046/j.1365-2249.1997.d01-907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobajima J., Ozaki S., Uesugi H., Osakada F., Shirakawa H., Yoshida M., Nakao K. Prevalence and characterization of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) directed against HMG1 and HMG2 in ulcerative colitis (UC). Clin Exp Immunol. 1998 Feb;111(2):402–407. doi: 10.1046/j.1365-2249.1998.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan S. R., Landers C., Vidrich A., Czaja A. J. High-titer antineutrophil cytoplasmic antibodies in type-1 autoimmune hepatitis. Gastroenterology. 1995 Apr;108(4):1159–1166. doi: 10.1016/0016-5085(95)90215-5. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., van der Woude F. J., Fauci A. S., Ambrus J. L., Velosa J., Keane W. F., Meijer S., van der Giessen M., van der Hem G. K., The T. H. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989 Nov;149(11):2461–2465. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- Uesugi H., Ozaki S., Sobajima J., Osakada F., Shirakawa H., Yoshida M., Nakao K. Prevalence and characterization of novel pANCA, antibodies to the high mobility group non-histone chromosomal proteins HMG1 and HMG2, in systemic rheumatic diseases. J Rheumatol. 1998 Apr;25(4):703–709. [PubMed] [Google Scholar]
- Vidrich A., Lee J., James E., Cobb L., Targan S. Segregation of pANCA antigenic recognition by DNase treatment of neutrophils: ulcerative colitis, type 1 autoimmune hepatitis, and primary sclerosing cholangitis. J Clin Immunol. 1995 Nov;15(6):293–299. doi: 10.1007/BF01541319. [DOI] [PubMed] [Google Scholar]
- Wen L., Huang J. K., Johnson B. H., Reeck G. R. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989 Feb 11;17(3):1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiik A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS Suppl. 1989;6:12–13. [PubMed] [Google Scholar]
- Yoshida M., Shimura K. Unwinding of DNA by nonhistone chromosomal protein HMG(1 + 2) from pig thymus as determined with endonuclease. J Biochem. 1984 Jan;95(1):117–124. doi: 10.1093/oxfordjournals.jbchem.a134573. [DOI] [PubMed] [Google Scholar]