Abstract
BACKGROUND/AIMS/PATIENTS—Glucocorticoid treatment is known to reduce nuclear factor-kappa B (NF-κB)p65 binding activity and activation in lamina propria cells of patients with Crohn's disease. However, lamina propria cells of glucocorticoid treated patients did not show increased expression of IκBα, and the hypothesised upregulation of IκBα by glucocorticoid treatment has not yet been shown in vivo. To investigate whether cells other than lamina propria localised mononuclear cells contribute to increased IκBα, resection gut specimens from patients matched for Crohn's disease activity index (CDAI) with or without glucocorticoid treatment were studied, and changes in the NF-κB/IκBα system were determined in the lamina propria as well as in underlying submucosal and endothelial cells. METHODS—Changes in the NF-κB/IκBα system were determined by immunohistochemistry, electrophoretic mobility shift assay, and western blot analysis in resected gut specimens from patients matched for CDAI and van Hees index with or without long term glucocorticoid treatment. RESULTS—Resection gut specimens from patients with Crohn's disease under glucocorticoid treatment had significantly lower nuclear NF-κBp65 levels in mononuclear, epithelial, and endothelial cells than samples from CDAI and van Hees index matched patients not having glucocorticoid treatment. Nuclear NF-κBp65 showed a strong positive correlation with both the CDAI (r = 1 for both groups) and the van Hees index (r = 0.605 for untreated and r = 0.866 for glucocorticoid treated specimens). Lower nuclear translocation of NF-κBp65 in the glucocorticoid treated group was paralleled by higher IκBα levels in vascular endothelial cells, but not in infiltrating mononuclear cells. CONCLUSION—A comparison of resection gut specimens from untreated and treated CDAI matched patients with Crohn's disease showed downregulation of NF-κB binding activity and NF-κBp65 expression and cell specific induction of endothelial IκBκ expression in the glucocorticoid treated group. As the two groups showed similar disease activity (CDAI, van Hees index), the activation of the NF-κBp65/IκBα system must be only part of the inflammatory cascade leading to the clinical appearance of Crohn's disease. Keywords: Crohn's disease; CDAI; inflammation; transcription factor; NF-κBp65; IκBα
Full Text
The Full Text of this article is available as a PDF (344.4 KB).
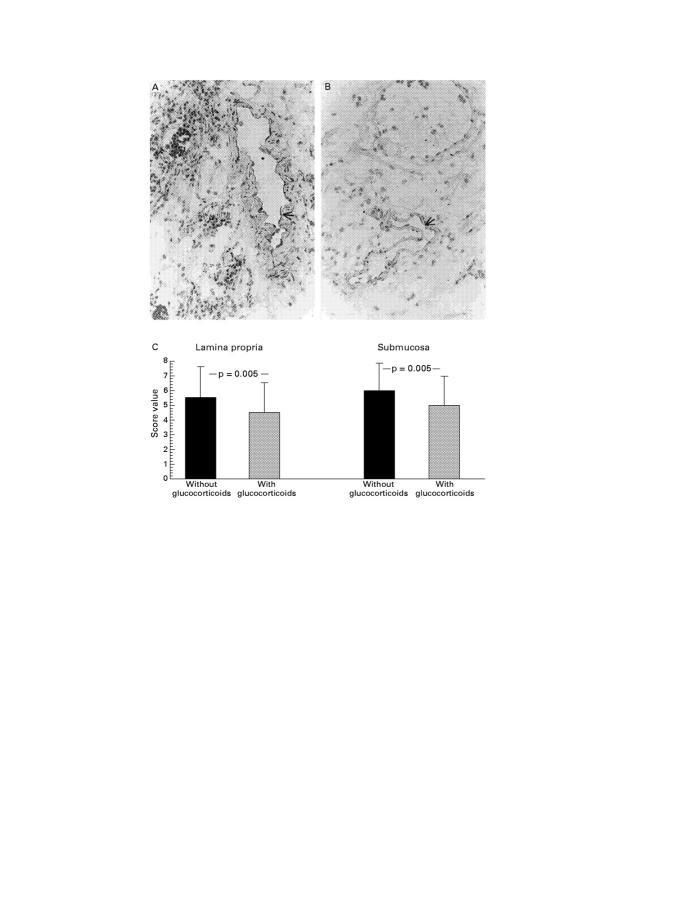
Figure 1 .
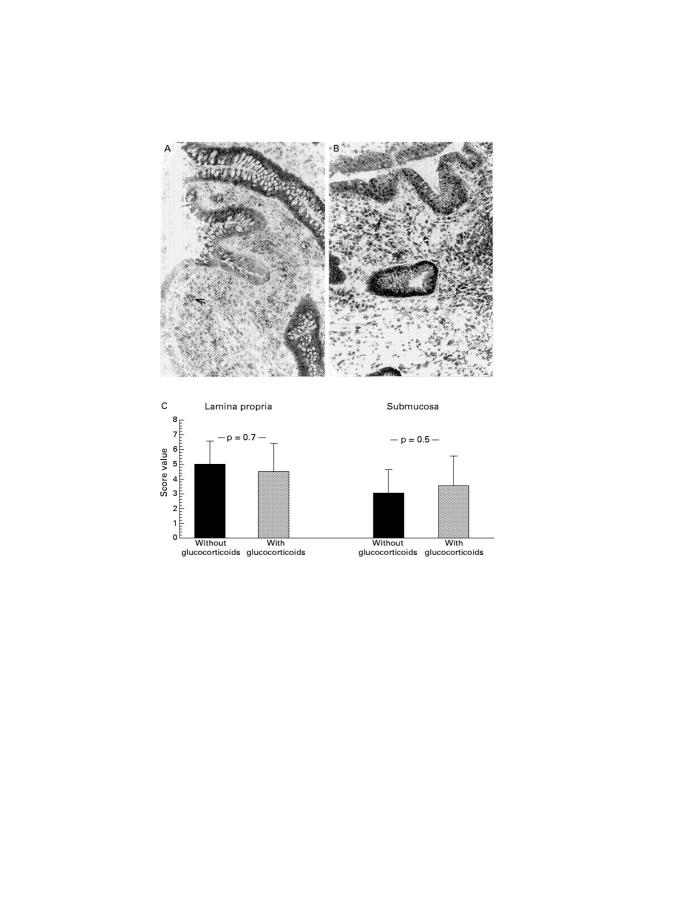
Immunohistochemical staining of activated NF-κBp65 antigen in resection gut specimens from patients with Crohn's disease. (A) Representative staining in a resection gut specimen from a patient without glucocorticoid treatment (original magnification × 40; representative positive stained cells are marked with an arrow). (B) Representative staining in a resection gut specimen from a patient with glucocorticoid treatment (original magnification × 40; representative positive stained cells are marked with an arrow). (C) Representative staining in a resection gut specimen from a patient without glucocorticoid treatment; counterstaining with haematoxylin was omitted (original magnification × 63). Many mononuclear cells in the lamina propria show nuclear staining. Some endothelial cells as well as crypt epithelial cells also express the molecule. (D) Representative staining in a resection gut specimen from a patient with glucocorticoid treatment; counterstaining with haematoxylin was omitted (original magnification × 100). Few mononuclear cells in the lamina propria show positive nuclear staining (arrows). Some additional positive cells display weak cytoplasmic expression of the molecule (arrowheads). (E) Summary of the scoring and the statistical results.
Figure 2 .
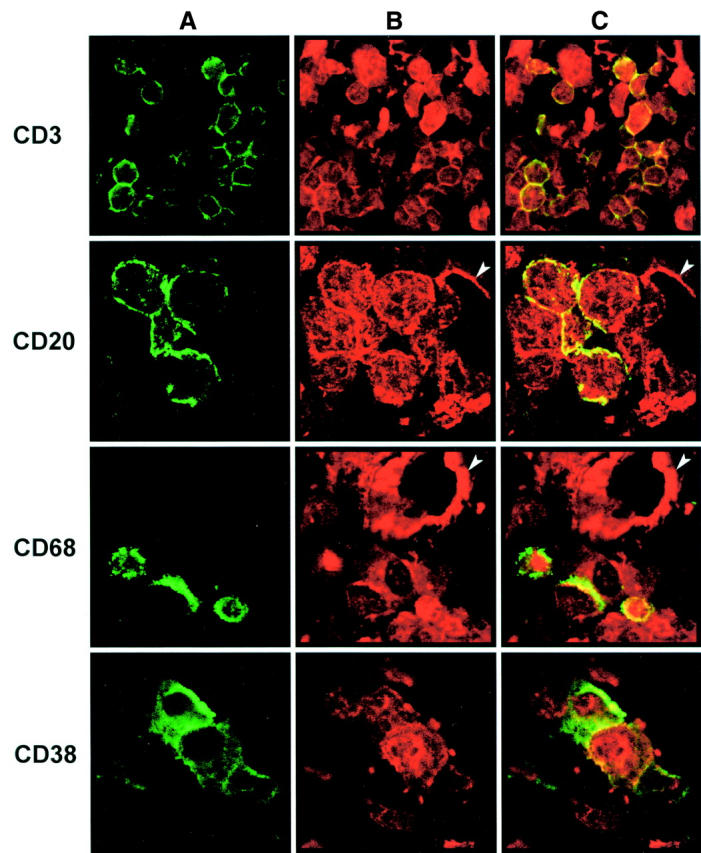
Double immunofluorescence staining of activated NF-κBp65 in combination with other cell markers in a resection gut specimen of a patient with Crohn's disease without glucocorticoid treatment. As leucocytes in the inflamed lamina propria show positive nuclear as well as cytoplasmic staining of NF-κBp65 (fig 1), simultaneous detection of CD3, CD20, CD68, or CD38 (column A; Cy2, green fluorescence) and NF-κBp65 (column B; Cy3, red fluorescence) was performed. This showed that NF-κBp65 is expressed by cells of all these leucocyte subsets. The double staining is shown in column C (Cy3, red; Cy2, green). Some vascular endothelial cells also express NF-κBp65 (arrowheads) (original magnifications: CD3, × 600; CD20, × 900; CD68, × 600; CD38, × 900).
Figure 3 .
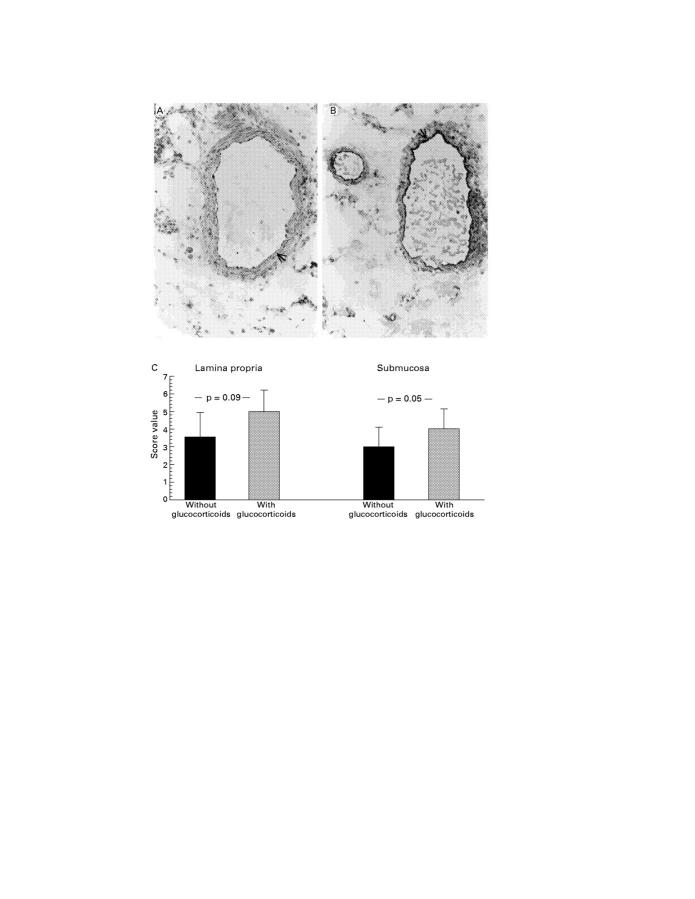
Immunohistochemical staining of activated NF-κBp65 antigen in resection gut specimens from patients with Crohn's disease (original magnification × 40); representative positive stained cells are marked with an arrow. (A) Representative staining of vascular endothelial cells in a resection gut specimen from a patient without glucocorticoid treatment. (B) Representative staining of vascular endothelial cells in a resection gut specimen from a patient with glucocorticoid treatment. (C) Summary of the scoring and the statistical results.
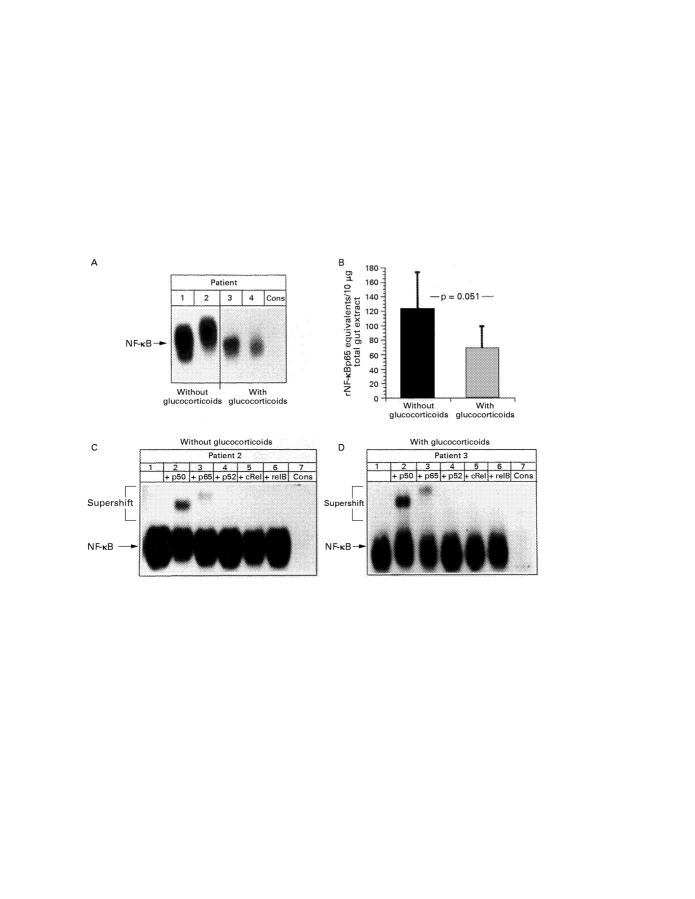
Figure 4 .
Total gut nuclear extract (10 µg) was prepared as described in Methods and assayed for NF-κB binding activity, monitored by electrophoretic mobility shift assay (EMSA). Radioactively labelled oligonucleotides spanning the consensus NF-κB recognition motif were incubated with equal amounts of nuclear extracts, and complexes were separated by electrophoresis on non-denaturing 5% polyacrylamide gels. (A) EMSA of four representative patients without (patients 1 and 2, lanes 1 and 2) and with (patients 3 and 4, lanes 3 and 4) glucocorticoid treatment. Specificity of NF-κB binding was shown by including a 160-fold molar excess of unlabelled consensus NF-κB oligonucleotide in the reaction with the extract from patient 2 (last lane). The position of NF-κB is indicated by an arrow. (B) Nuclear extracts of all resection gut specimens (table 1) were assayed for NF-κB binding activity by EMSA. Intensity of signals was expressed as recombinant NF-κBp65 (rNF-κBp65) equivalents using an internal standard curve for rNF-κBp65 (data not shown41). (C,D) Characterisation of the NF-κB subunits contributing to the observed shift formed at the NF-κB consensus sequence was performed by including 2.5 µg of anti-p50 (lane 2), anti-p65 (lane 3), anti-p52 (lane 4), anti-cRel (lane 5), or anti-relB (lane 6) antibodies in the binding reactions with nuclear extracts of a Crohn's disease patient without (C) or with (D) glucocorticoid treatment. Specificity of NF-κB binding was shown by including a 160-fold molar excess of unlabelled consensus NF-κB oligonucleotide in the reaction with extract from patient 2 (lane 7). The position of NF-κB is indicated by arrows.
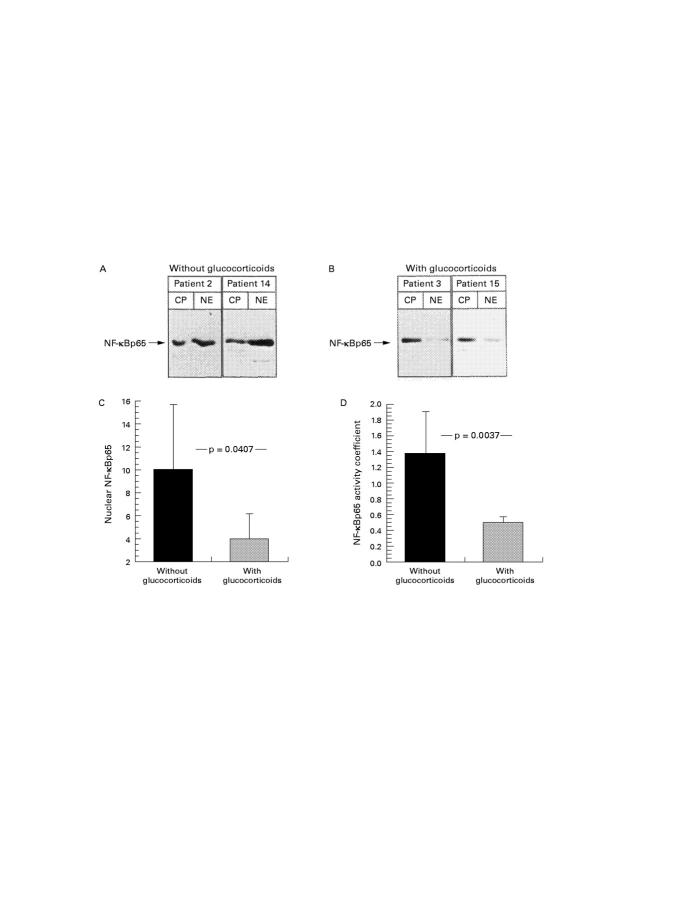
Figure 5 .
(A, B) Cytoplasmic (CP) and nuclear (NE) extracts were prepared from resection gut specimens of two patients without (patients 2 and 14; A) and two with (patients 3 and 15; B) glucocorticoid treatment as described in the Methods. Fractions were separated by SDS/PAGE (12.5% gel) and subjected to immunoblotting with an antibody for NF-κBp65. Horseradish peroxidase coupled antibodies were used as secondary antibodies, and signals were detected with the ECL-western blot detection system. The specific complexes are indicated by arrows. (C) Nuclear NF-κBp65 detected by western blot in samples without (n = 10) and with (n = 5) glucocorticoid treatment was evaluated by densitometry. The determination of the signal area to be measured and the quantitative evaluation was performed twice for two independent experiments. The mean of the two measurements was taken for statistical analysis. (D) To adjust for individual differences in NF-κB expression, the ratio of nuclear to cytoplasmic NF-κBp65 (table 4) determined in western blot analysis was calculated to give an NF-κB activity coefficient for resection gut specimens without and with glucocorticoid treatment.
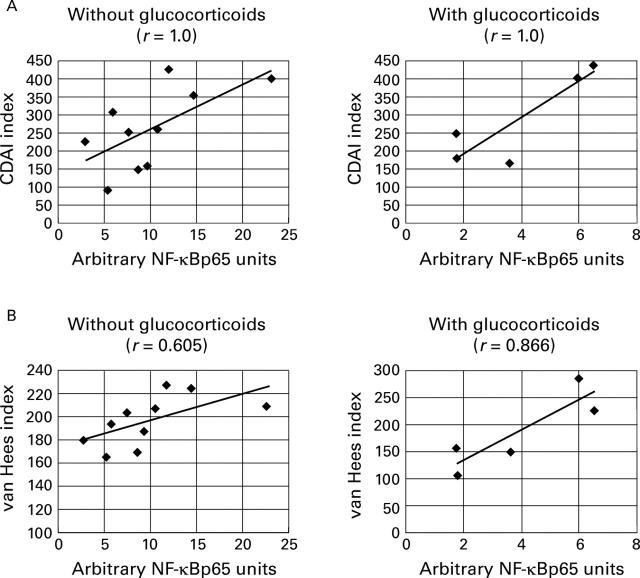
Figure 6 .
Correlation of nuclear NF-κBp65 (table 4) with (A) Crohn's disease activity index and (B) van Hees index in patients without and with glucocorticoid treatment.
Figure 7 .
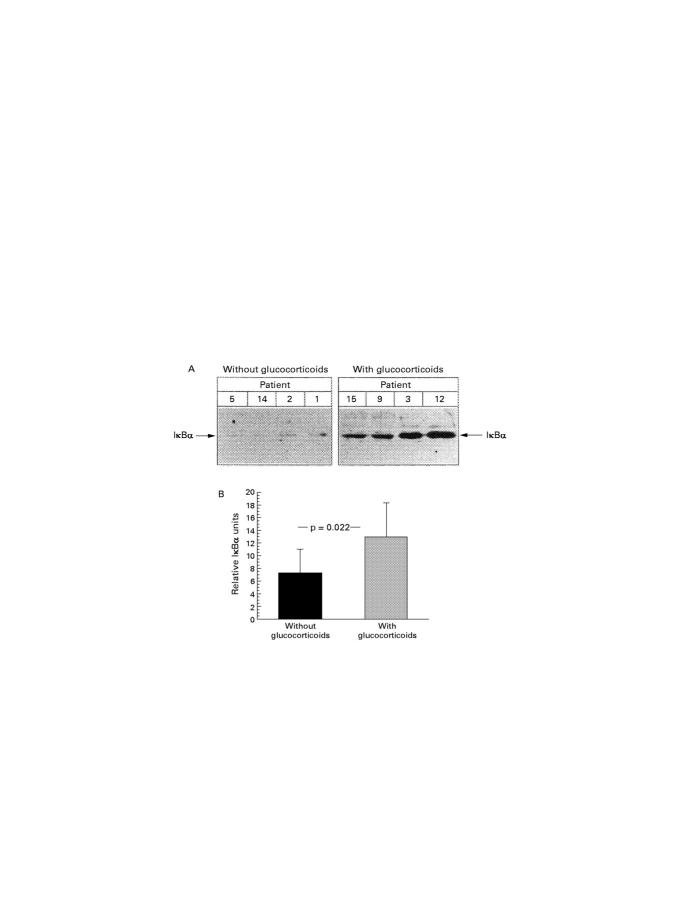
(A) Cytoplasmic extracts were prepared from resection gut specimens from four patients without (patient 5, 14, 2, 1; left) and four patients with (patients 15, 9, 3, 12; right) glucocorticoid treatment as described in the Methods. Fractions were separated by SDS/PAGE (12.5% gel) and subjected to immunoblotting with an antibody for full length IκBα. Horseradish peroxidase coupled antibodies were used as secondary antibodies and signals were detected with the ECL-western blot detection system. The specific complexes are indicated by arrows. (B) Cytoplasmic IκBα detected by western blot analysis in samples without (n = 10) and with (n = 5) glucocorticoid treatment was evaluated by densitometry. Determination of the signal area to be measured and the quantitative evaluation was performed twice for two independent experiments. The mean of the two measurements was taken for statistical analysis.
Figure 8 .
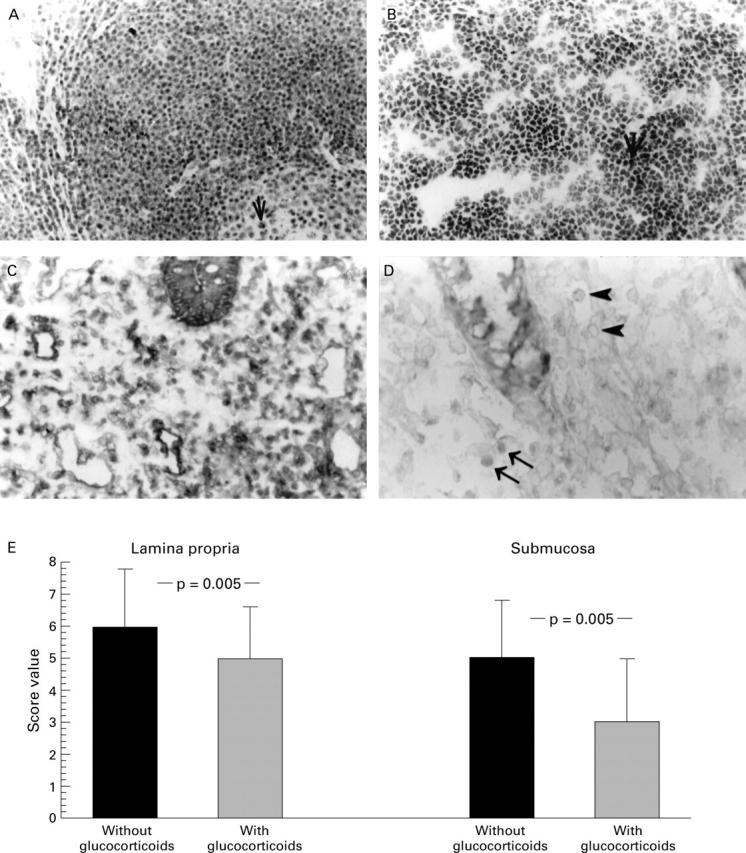
Immunohistochemical staining of IκBα antigen in resection gut specimens from patients with Crohn's disease (original magnification × 40); representative positive stained cells are marked with an arrow. (A) Representative staining of mononuclear cells in a resection gut specimen from a patient without glucocorticoid treatment. (B) Representative staining of mononuclear cells in a resection gut specimen from a patient with glucocorticoid treatment. (C) Summary of the scoring and the statistical results.
Figure 9 .
Immunohistochemical staining of IκBα antigen in resection gut specimens of patients with Crohn's disease (original magnification × 40); representative positive stained cells are marked with an arrow. (A) Representative staining of vascular endothelial cells in a resection gut specimen from a patient without glucocorticoid treatment. (B) Representative staining of vascular endothelial cells in a resection gut specimen from a patient with glucocorticoid treatment. (C) Summary of the scoring and the statistical results.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardite E., Panés J., Miranda M., Salas A., Elizalde J. I., Sans M., Arce Y., Bordas J. M., Fernández-Checa J. C., Piqué J. M. Effects of steroid treatment on activation of nuclear factor kappaB in patients with inflammatory bowel disease. Br J Pharmacol. 1998 Jun;124(3):431–433. doi: 10.1038/sj.bjp.0701887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auphan N., DiDonato J. A., Rosette C., Helmberg A., Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995 Oct 13;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baichwal V. R. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–137. [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. NF-kappa B: ten years after. Cell. 1996 Oct 4;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997 Apr 10;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baldwin A. S., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993 Nov;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Bierhaus A., Illmer T., Kasper M., Luther T., Quehenberger P., Tritschler H., Wahl P., Ziegler R., Müller M., Nawroth P. P. Advanced glycation end product (AGE)-mediated induction of tissue factor in cultured endothelial cells is dependent on RAGE. Circulation. 1997 Oct 7;96(7):2262–2271. doi: 10.1161/01.cir.96.7.2262. [DOI] [PubMed] [Google Scholar]
- Bierhaus A., Zhang Y., Deng Y., Mackman N., Quehenberger P., Haase M., Luther T., Müller M., Böhrer H., Greten J. Mechanism of the tumor necrosis factor alpha-mediated induction of endothelial tissue factor. J Biol Chem. 1995 Nov 3;270(44):26419–26432. doi: 10.1074/jbc.270.44.26419. [DOI] [PubMed] [Google Scholar]
- Bierhaus A., Zhang Y., Quehenberger P., Luther T., Haase M., Müller M., Mackman N., Ziegler R., Nawroth P. P. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997 Apr;77(4):772–782. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brostjan C., Anrather J., Csizmadia V., Stroka D., Soares M., Bach F. H., Winkler H. Glucocorticoid-mediated repression of NFkappaB activity in endothelial cells does not involve induction of IkappaBalpha synthesis. J Biol Chem. 1996 Aug 9;271(32):19612–19616. doi: 10.1074/jbc.271.32.19612. [DOI] [PubMed] [Google Scholar]
- Böhrer H., Qiu F., Zimmermann T., Zhang Y., Jllmer T., Männel D., Böttiger B. W., Stern D. M., Waldherr R., Saeger H. D. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997 Sep 1;100(5):972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldenhoven E., Liden J., Wissink S., Van de Stolpe A., Raaijmakers J., Koenderman L., Okret S., Gustafsson J. A., Van der Saag P. T. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol. 1995 Apr;9(4):401–412. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- Chapple I. L. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997 May;24(5):287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Cohen L., Henzel W. J., Baeuerle P. A. IKAP is a scaffold protein of the IkappaB kinase complex. Nature. 1998 Sep 17;395(6699):292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- Collins T. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 1993 May;68(5):499–508. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- De Bosscher K., Schmitz M. L., Vanden Berghe W., Plaisance S., Fiers W., Haegeman G. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. D., Limb G. A., Thompson R. P., Punchard N. A. NF kappa B in Crohn's disease. Biochem Soc Trans. 1997 May;25(2):178S–178S. doi: 10.1042/bst025178s. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier D. L., Miner P. B., Jr New therapeutic agents in the treatment of inflammatory bowel disease. Am J Med. 1992 Aug;93(2):199–208. doi: 10.1016/0002-9343(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Heck S., Bender K., Kullmann M., Göttlicher M., Herrlich P., Cato A. C. I kappaB alpha-independent downregulation of NF-kappaB activity by glucocorticoid receptor. EMBO J. 1997 Aug 1;16(15):4698–4707. doi: 10.1093/emboj/16.15.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt C., Kaltschmidt B., Henkel T., Stockinger H., Baeuerle P. A. Selective recognition of the activated form of transcription factor NF-kappa B by a monoclonal antibody. Biol Chem Hoppe Seyler. 1995 Jan;376(1):9–16. doi: 10.1515/bchm3.1995.376.1.9. [DOI] [PubMed] [Google Scholar]
- Keatings V. M., Jatakanon A., Worsdell Y. M., Barnes P. J. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997 Feb;155(2):542–548. doi: 10.1164/ajrccm.155.2.9032192. [DOI] [PubMed] [Google Scholar]
- Lechner J., Welte T., Tomasi J. K., Bruno P., Cairns C., Gustafsson J., Doppler W. Promoter-dependent synergy between glucocorticoid receptor and Stat5 in the activation of beta-casein gene transcription. J Biol Chem. 1997 Aug 15;272(33):20954–20960. doi: 10.1074/jbc.272.33.20954. [DOI] [PubMed] [Google Scholar]
- Levin M. E. Diabetes and peripheral neuropathy. Diabetes Care. 1998 Jan;21(1):1–1. doi: 10.2337/diacare.21.1.1. [DOI] [PubMed] [Google Scholar]
- McKay L. I., Cidlowski J. A. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol. 1998 Jan;12(1):45–56. doi: 10.1210/mend.12.1.0044. [DOI] [PubMed] [Google Scholar]
- Neurath M. F. Pathogenesis of inflammatory bowel disease: transcription factors in the spotlight. Gut. 1998 Apr;42(4):458–459. doi: 10.1136/gut.42.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Pettersson S., Meyer zum Büschenfelde K. H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996 Sep;2(9):998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Neurath M. F., Pettersson S. Predominant role of NF-kappa B p65 in the pathogenesis of chronic intestinal inflammation. Immunobiology. 1997 Dec;198(1-3):91–98. doi: 10.1016/s0171-2985(97)80030-7. [DOI] [PubMed] [Google Scholar]
- Norbiato G., Bevilacqua M., Vago T., Clerici M. Glucocorticoids and Th-1, Th-2 type cytokines in rheumatoid arthritis, osteoarthritis, asthma, atopic dermatitis and AIDS. Clin Exp Rheumatol. 1997 May-Jun;15(3):315–323. [PubMed] [Google Scholar]
- Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T., Knuechel R., Baeuerle P. A., Schölmerich J., Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998 Aug;115(2):357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Rothwarf D. M., Zandi E., Natoli G., Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998 Sep 17;395(6699):297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- Scheinman R. I., Cogswell P. C., Lofquist A. K., Baldwin A. S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995 Oct 13;270(5234):283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- Scheinman R. I., Gualberto A., Jewell C. M., Cidlowski J. A., Baldwin A. S., Jr Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995 Feb;15(2):943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S., Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998 Apr;42(4):477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainer B., Nielsen O. H. Adhaesionsmolekylers betydning ved inflammatorisk tarmsygdom. Ugeskr Laeger. 1997 Jun 9;159(24):3767–3771. [PubMed] [Google Scholar]
- Vayssière B. M., Dupont S., Choquart A., Petit F., Garcia T., Marchandeau C., Gronemeyer H., Resche-Rigon M. Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo. Mol Endocrinol. 1997 Aug;11(9):1245–1255. doi: 10.1210/mend.11.9.9979. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Stevenson J. K., Schwarz E. M., Van Antwerp D., Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995 Nov 15;9(22):2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Wahl C., Liptay S., Adler G., Schmid R. M. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998 Mar 1;101(5):1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens T., De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today. 1997 Sep;18(9):418–424. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- Wisniewska M., Stanczyk M., Grzelakowska-Sztabert B., Kaminska B. Nuclear factor of activated T cells (NFAT) is a possible target for dexamethasone in thymocyte apoptosis. Cell Biol Int. 1997 Mar;21(3):127–132. doi: 10.1006/cbir.1996.0124. [DOI] [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]