Abstract
BACKGROUND—The intestinal mucosa harbours a large number of nerve fibres and also plasma cells, providing the anatomical basis for studies of neuroimmune interactions. AIMS—To study the effect of different neurotransmitters and electrical stimulation of the extrinsic intestinal nerves on secretion of immunoglobulin A (IgA). METHODS—IgA was measured, using a specific ELISA, in the luminal and venous effluent from isolated vascularly perfused porcine ileal segments with preserved extrinsic nerve supply. RESULTS—Infusion of several neuropeptides stimulated IgA output. Somatostatin (10−8 M) stimulated IgA secretion in the luminal effluent from 46.6 (14.3) to 79.3 (19.0) µg/5 min and increased the venous output to 148.3 (23.0)% (n=6) of basal output, whereas noradrenaline (10−6 M) inhibited the secretion (to 49.2 (6.5)% of basal output, n=6). Electrical stimulation of the mixed extrinsic nerves supplying the intestinal segment had no effect by itself. However, electrical stimulation during infusion of α adrenergic blockers or coinfusion of both α adrenergic and muscarinic blockers resulted in an immediate and significant increase in IgA, an effect that was abolished by nicotinic blockade. CONCLUSION—The extrinsic nerve supply to the intestine could be involved in fast acting regulation of mucosal immune functions. Keywords: neuroimmunology; enteric nervous system; neuropeptide; parasympathetic; sympathetic
Full Text
The Full Text of this article is available as a PDF (120.0 KB).
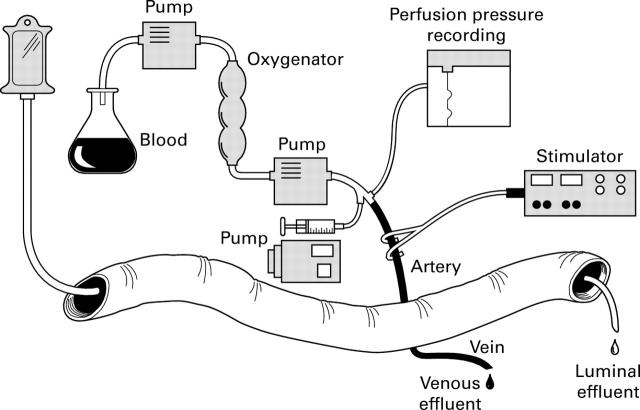
Figure 1 .
Illustration of the isolated vascularly perfused porcine ileum placed in a thermostatically controlled bath (37°C). The segment receives perfusion medium containing oxygenated erythrocytes through the ileal artery. Perfusion flow is kept constant and the perfusion pressure measured continuously. The extrinsic nerves to the segment situated around the artery can be electrically stimulated. The lumen of the segment is perfused at a constant rate (not illustrated) and both luminal effluent and the venous effluent are collected.
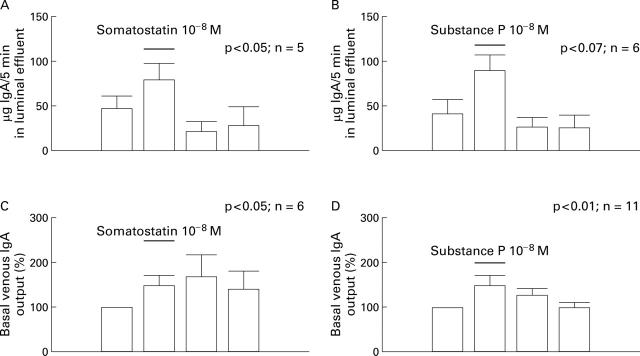
Figure 2 .
Effects of infusions of substance P and somatostatin (both 10−8 M) on release of IgA to the lumen (upper panel) and the venous effluent (lower panel). Results expressed as mean (SEM). The four bars represent the prestimulatory, stimulatory, and two poststimulatory five minute periods.
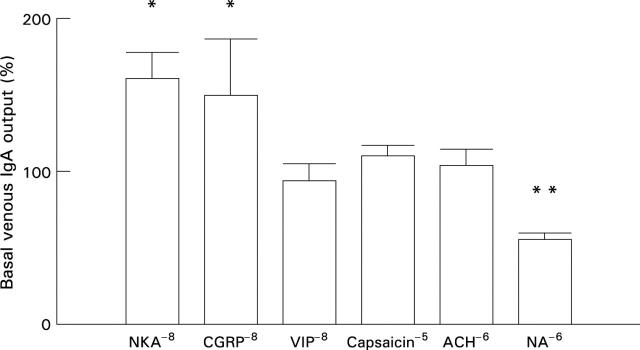
Figure 3 .
Effects of neurokinin A (NKA), calcitonin gene related peptide (CGRP), vasoactive intestinal polypeptide (VIP), capsaicin, acetylcholine (ACH), and noradrenaline (NA) on the release of IgA to the venous effluent. Results expressed as mean (SEM). *p<0.05, **p<0.01.
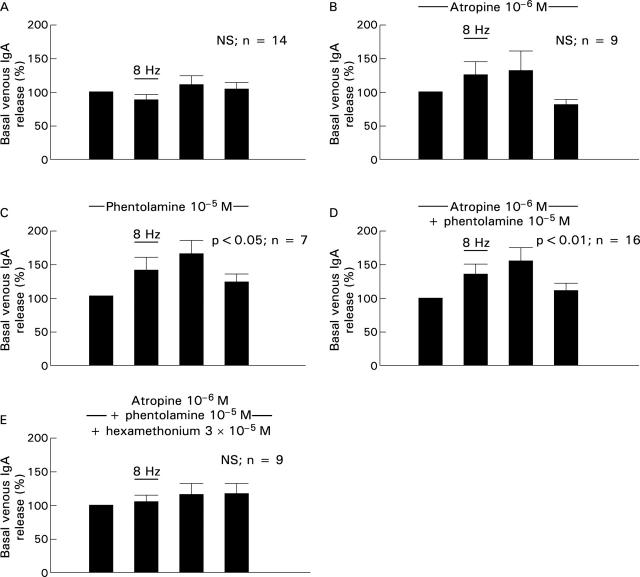
Figure 4 .
Effect of electrical stimulation of the extrinsic nerves at 8 Hz on the release of IgA to the venous effluent under basal conditions and during infusion of different receptor antagonists, expressed as percentage of basal release. Results expressed as mean (SEM). The four bars represent the prestimulatory, stimulatory, and two poststimulatory five minute periods.
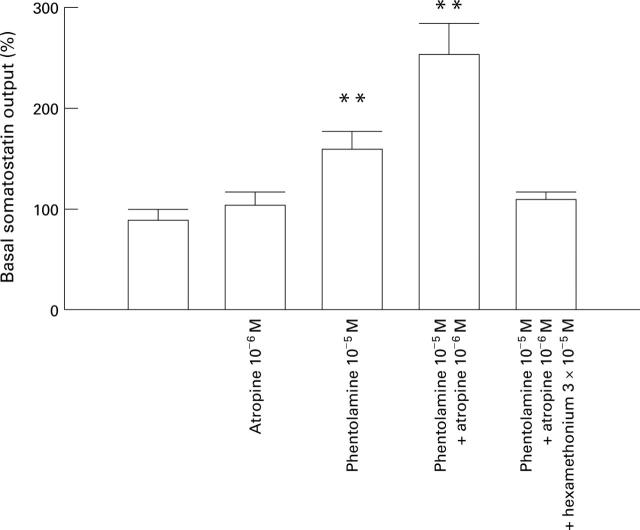
Figure 5 .
Effect of electrical stimulation of the extrinsic nerves at 8 Hz during infusion of different receptor antagonists on somatostatin release to the venous effluent. Results expressed as mean (SEM). **p<0.01.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader R., Cohen N., Felten D. L. Brain, behavior, and immunity. Brain Behav Immun. 1987 Mar;1(1):1–6. doi: 10.1016/0889-1591(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Bellinger D. L., Lorton D., Felten S. Y., Felten D. L. Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol. 1992 Apr;14(3):329–344. doi: 10.1016/0192-0561(92)90162-e. [DOI] [PubMed] [Google Scholar]
- Boirivant M., Fais S., Annibale B., Agostini D., Delle Fave G., Pallone F. Vasoactive intestinal polypeptide modulates the in vitro immunoglobulin A production by intestinal lamina propria lymphocytes. Gastroenterology. 1994 Mar;106(3):576–582. doi: 10.1016/0016-5085(94)90688-2. [DOI] [PubMed] [Google Scholar]
- Calvo J. R., Montilla M. L., Guerrero J. M., Segura J. J. Expression of VIP receptors in mouse peritoneal macrophages: functional and molecular characterization. J Neuroimmunol. 1994 Feb;50(1):85–93. doi: 10.1016/0165-5728(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Carucci J. A., Herrick C. A., Durkin H. G. Neuropeptide-mediated regulation of hapten-specific IgE responses in mice. II. Mechanisms of substance P-mediated isotype-specific suppression of BPO-specific IgE antibody-forming cell responses induced in vitro. J Neuroimmunol. 1994 Jan;49(1-2):89–95. doi: 10.1016/0165-5728(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Fehér E., Fodor M., Burnstock G. Distribution of somatostatin-immunoreactive nerve fibres in Peyer's patches. Gut. 1992 Sep;33(9):1195–1198. doi: 10.1136/gut.33.9.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier S., Eran M., Alon Y., Elath U. Verapamil and furosemide prevent cholecystokinin-induced translocation of immunoglobulins in rat intestine. Dig Dis Sci. 1991 Nov;36(11):1619–1624. doi: 10.1007/BF01296407. [DOI] [PubMed] [Google Scholar]
- Freier S., Eran M., Faber J. Effect of cholecystokinin and of its antagonist, of atropine, and of food on the release of immunoglobulin A and immunoglobulin G specific antibodies in the rat intestine. Gastroenterology. 1987 Dec;93(6):1242–1246. doi: 10.1016/0016-5085(87)90251-4. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Wolters K., Toyka K. V. Substance P: binding properties and studies on cellular responses in guinea pig macrophages. J Immunol. 1986 May 15;136(10):3856–3863. [PubMed] [Google Scholar]
- Helme R. D., Eglezos A., Dandie G. W., Andrews P. V., Boyd R. L. The effect of substance P on the regional lymph node antibody response to antigenic stimulation in capsaicin-pretreated rats. J Immunol. 1987 Nov 15;139(10):3470–3473. [PubMed] [Google Scholar]
- Hilsted L., Holst J. J. On the accuracy of radioimmunological determination of somatostatin in plasma. Regul Pept. 1982 Jun;4(1):13–31. doi: 10.1016/0167-0115(82)90105-7. [DOI] [PubMed] [Google Scholar]
- Ichikawa S., Sreedharan S. P., Goetzl E. J., Owen R. L. Immunohistochemical localization of peptidergic nerve fibers and neuropeptide receptors in Peyer's patches of the cat ileum. Regul Pept. 1994 Dec 15;54(2-3):385–395. doi: 10.1016/0167-0115(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Kimata H., Yoshida A., Ishioka C., Fujimoto M., Furusho K. Vasoactive intestinal peptide enhances immunoglobulin production and growth in human plasma cells via mechanisms that may involve protein kinase C. J Clin Endocrinol Metab. 1996 Aug;81(8):3024–3032. doi: 10.1210/jcem.81.8.8768869. [DOI] [PubMed] [Google Scholar]
- Loftager M. K., Eriksen L., Nielsen R. Antibodies against Actinobacillus pleuropneumoniae serotype 2 in mucosal secretions and sera of infected pigs as demonstrated by an enzyme-linked immunosorbent assay. Res Vet Sci. 1993 Jan;54(1):57–62. doi: 10.1016/0034-5288(93)90011-4. [DOI] [PubMed] [Google Scholar]
- Messell T., Harling H., Poulsen S. S., Bersani M., Holst J. J. Extrinsic control of the release of galanin and VIP from intrinsic nerves of isolated, perfused, porcine ileum. Regul Pept. 1992 Apr 9;38(3):179–198. doi: 10.1016/0167-0115(92)90101-y. [DOI] [PubMed] [Google Scholar]
- Pascual D. W., Bost K. L., Xu-Amano J., Kiyono H., McGhee J. R. The cytokine-like action of substance P upon B cell differentiation. Reg Immunol. 1992 Mar-Apr;4(2):100–104. [PubMed] [Google Scholar]
- Rasmussen T. N., Bersani M., Schmidt P., Thim L., Kofod H., Jørgensen P. N., Poulsen S. S., Holst J. J. Isolation and molecular characterization of porcine calcitonin gene-related peptide (CGRP) and its endocrine effects in the porcine pancreas. Pancreas. 1998 Mar;16(2):195–204. doi: 10.1097/00006676-199803000-00014. [DOI] [PubMed] [Google Scholar]
- Schmidt P., Rasmussen T. N., Holst J. J. Nervous control of the release of substance P and neurokinin A from the isolated perfused porcine ileum. J Auton Nerv Syst. 1992 May 1;38(2):85–95. doi: 10.1016/0165-1838(92)90229-a. [DOI] [PubMed] [Google Scholar]
- Schmidt P., Rasmussen T. N., Holst J. J. Release of immunoreactive somatostatin, vasoactive intestinal polypeptide (VIP), and galanin during propulsive complexes in isolated pig ileum. Peptides. 1993 Mar-Apr;14(2):215–220. doi: 10.1016/0196-9781(93)90032-c. [DOI] [PubMed] [Google Scholar]
- Stanisz A. M., Befus D., Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986 Jan;136(1):152–156. [PubMed] [Google Scholar]
- Stanisz A. M., Scicchitano R., Dazin P., Bienenstock J., Payan D. G. Distribution of substance P receptors on murine spleen and Peyer's patch T and B cells. J Immunol. 1987 Aug 1;139(3):749–754. [PubMed] [Google Scholar]
- Thorbøll J. E., Bindslev N., Tindholdt T. T., Schmidt P., Christensen P., Skadhauge E. Tachykinins mediate changes in ion transport in porcine jejunum through release of prostaglandins and neurotransmitters. Regul Pept. 1998 Oct 16;77(1-3):105–111. doi: 10.1016/s0167-0115(98)00106-2. [DOI] [PubMed] [Google Scholar]
- Wilson I. D., Soltis R. D., Olson R. E., Erlandsen S. L. Cholinergic stimulation of immunoglobulin A secretion in rat intestine. Gastroenterology. 1982 Oct;83(4):881–888. [PubMed] [Google Scholar]