Abstract
BACKGROUND—It is not known whether cagA+ Helicobacter pylori in duodenal ulcer (DU) have enhanced virulence compared with non-DU cagA+ H pylori. AIMS—To investigate the relation between presentation, H pylori density, interleukin 1β (IL-1β) and IL-8 production, and cagA status. METHODS—Fifty DU and 50 gastritis patients with cagA+ H pylori and 11 with cagA− infections were studied. Bacterial density and cytokine production were assessed using the same biopsies. Cytokine production was also measured from supernatants of medium following coculture of H pylori with MKN-45 cells. RESULTS—There was no relation between H pylori density and cagA status. There was a dose dependent relation between mucosal cytokine levels and density of cagA+ H pylori. H pylori density increased to a threshold, followed by a rapid increase in cytokines and then a plateau. IL-1β and IL-8 levels in the antrum were greater in DU than in gastritis; in the corpus the cytokine level/H pylori differed irrespective of similar H pylori densities. However, cytokine production was similar in vitro, independent of presentation or biopsy site, suggesting that host factors are critical determinants of the inflammatory response. Mucosal IL-8 and IL-1β levels were low with cagA− and cagA+, cagE− H pylori infections. CONCLUSIONS—The increase in antral IL-1β and IL-8 production and inflammation in DU is related to increased numbers of bacteria and not to an increase in cytokine production per cagA+ isolate. There was no evidence of enhanced virulence of H pylori from DU compared with cagA+ non-DU H pylori. Keywords: duodenal ulcer; Helicobacter pylori; interleukin 1β; interleukin 8; cagA
Full Text
The Full Text of this article is available as a PDF (155.3 KB).
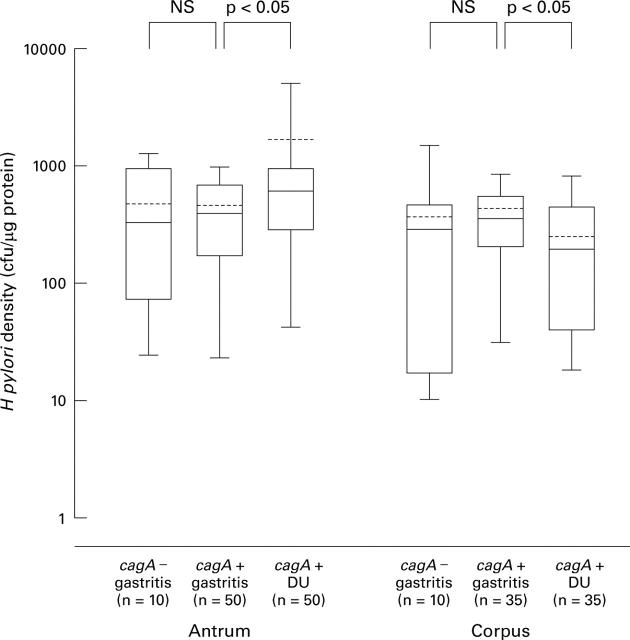
Figure 1 .
Helicobacter pylori density in antrum and corpus. The end of the bars indicates the 25th and 75th percentiles. The 50th percentile (median) is indicated with a solid line in the box; the broken line indicates mean value. The 10th and 90th percentiles are indicated with error bars. DU, duodenal ulcer.
Figure 2 .
Relation between Helicobacter pylori density by culture and cellular infiltration in cagA+ cases. DU, duodenal ulcer; MNC, mononuclear cell; PMN, polymorphonuclear cell.
Figure 3 .
Mucosal interleukin (IL) 8 production. The end of the bars indicates the 25th and 75th percentiles. The 50th percentile (median) is indicated with a line in the box and the 10th and 90th percentiles are indicated with error bars. *Two cagA+ gastritis cases with extremely low in vitro IL-8 production, which indicate cagA positive, cagE, cagG negative strains. DU, duodenal ulcer.
Figure 4 .
Relation between mucosal interleukin (IL) 1β and IL-8 production and Helicobacter pylori density of cagA+ strains in the antrum.
Figure 5 .
Relation between mucosal interleukin (IL) 1β and IL-8 production and Helicobacter pylori density of cagA+ strains in the corpus.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton J. C., Cao P., Peek R. M., Jr, Tummuru M. K., Blaser M. J., Cover T. L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995 Jul 28;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Tham K. T., Peek R. M., Jr, Cover T. L., Blaser M. J. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996 Sep;174(3):552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Xiang Z., Lindley I. J., Tompkins D. S., Rappuoli R., Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995 Oct;48(10):967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues J. V., Martínez-Cuesta M. A., Barrachina M. D., Calatayud S., Whittle B. J. Involvement of endogenous nitric oxide in the inhibition by endotoxin and interleukin-1 beta of gastric acid secretion. J Gastroenterol Hepatol. 1994;9 (Suppl 1):S45–S49. doi: 10.1111/j.1440-1746.1994.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997 Dec;113(6):1983–1991. doi: 10.1016/s0016-5085(97)70019-2. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Yamaoka Y. H. pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998 Sep;3(3):145–151. doi: 10.1046/j.1523-5378.1998.08031.x. [DOI] [PubMed] [Google Scholar]
- Khulusi S., Mendall M. A., Patel P., Levy J., Badve S., Northfield T. C. Helicobacter pylori infection density and gastric inflammation in duodenal ulcer and non-ulcer subjects. Gut. 1995 Sep;37(3):319–324. doi: 10.1136/gut.37.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Shinomura Y., Kanayama S., Higashimoto Y., Kiyohara T., Yasunaga Y., Kitamura S., Ueyama H., Imamura I., Fukui H. Helicobacter pylori increases gene expression of hepatocyte growth factor in human gastric mucosa. Biochem Biophys Res Commun. 1995 May 25;210(3):960–965. doi: 10.1006/bbrc.1995.1750. [DOI] [PubMed] [Google Scholar]
- Kondo S., Shinomura Y., Kanayama S., Kawabata S., Miyazaki Y., Imamura I., Fukui H., Matsuzawa Y. Interleukin-1 beta inhibits gastric histamine secretion and synthesis in the rat. Am J Physiol. 1994 Dec;267(6 Pt 1):G966–G971. doi: 10.1152/ajpgi.1994.267.6.G966. [DOI] [PubMed] [Google Scholar]
- Maeda S., Yoshida H., Ikenoue T., Ogura K., Kanai F., Kato N., Shiratori Y., Omata M. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut. 1999 Mar;44(3):336–341. doi: 10.1136/gut.44.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R. M., Jr, Thompson S. A., Donahue J. P., Tham K. T., Atherton J. C., Blaser M. J., Miller G. G. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians. 1998 Nov-Dec;110(6):531–544. [PubMed] [Google Scholar]
- Sharma S. A., Tummuru M. K., Miller G. G., Blaser M. J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995 May;63(5):1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- Tunio A. M., Holton J., Hobsley M. Gastric juice epidermal growth factor concentration and Helicobacter pylori in patients with duodenal ulcer. Br J Surg. 1995 Sep;82(9):1204–1206. doi: 10.1002/bjs.1800820917. [DOI] [PubMed] [Google Scholar]
- Uemura N., Oomoto Y., Mukai T., Okamoto S., Yamaguchi S., Mashiba H., Taniyama K., Sasaki N., Sumii K., Haruma K. Gastric corpus IL-8 concentration and neutrophil infiltration in duodenal ulcer patients. Aliment Pharmacol Ther. 1997 Aug;11(4):793–800. doi: 10.1046/j.1365-2036.1997.00218.x. [DOI] [PubMed] [Google Scholar]
- Warburton V. J., Everett S., Mapstone N. P., Axon A. T., Hawkey P., Dixon M. F. Clinical and histological associations of cagA and vacA genotypes in Helicobacter pylori gastritis. J Clin Pathol. 1998 Jan;51(1):55–61. doi: 10.1136/jcp.51.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996 Jun;110(6):1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Kashima K., Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997 Oct;41(4):442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Tanahashi T., Kashima K., Imanishi J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998 May;42(5):609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Graham D. Y., Kashima K. Search for putative virulence factors of Helicobacter pylori: the low-molecular-weight (33-35 K) antigen. Dig Dis Sci. 1998 Jul;43(7):1482–1487. doi: 10.1023/a:1018850412148. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Kashima K., Graham D. Y., Sepulveda A. R. Variants of the 3' region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol. 1998 Aug;36(8):2258–2263. doi: 10.1128/jcm.36.8.2258-2263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Kita M., Imanishi J., Kashima K., Graham D. Y. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998 Dec;3(4):241–253. doi: 10.1046/j.1523-5378.1998.08056.x. [DOI] [PubMed] [Google Scholar]
- Yasunaga Y., Shinomura Y., Kanayama S., Higashimoto Y., Yabu M., Miyazaki Y., Murayama Y., Nishibayashi H., Kitamura S., Matsuzawa Y. Mucosal interleukin-1 beta production and acid secretion in enlarged fold gastritis. Aliment Pharmacol Ther. 1997 Aug;11(4):801–809. doi: 10.1046/j.1365-2036.1997.00200.x. [DOI] [PubMed] [Google Scholar]
- el-Zimaity H. M., Graham D. Y., al-Assi M. T., Malaty H., Karttunen T. J., Graham D. P., Huberman R. M., Genta R. M. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996 Jan;27(1):35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.