Abstract
BACKGROUND—Liver/kidney microsomal antibody type 1 (LKM1) is the marker of type 2 autoimmune hepatitis (AIH) and is detected in up to 6% of patients with hepatitis C virus (HCV) infection. It recognises linear and conformational epitopes of cytochrome P450IID6 (CYP2D6) and may have liver damaging activity, provided that CYP2D6 is accessible to effector mechanisms of autoimmune attack. METHODS—The presence of LKM1 in the plasma membrane was investigated by indirect immunofluorescence and confocal laser microscopy of isolated rat hepatocytes probed with 10 LKM1 positive sera (five from patients with AIH and five from patients with chronic HCV infection) and a rabbit polyclonal anti-CYP2D6 serum. RESULTS—Serum from both types of patient stained the plasma membrane of non-permeabilised cells, where the fluorescent signal could be visualised as discrete clumps. Conversely, permeabilised hepatocytes showed diffuse submembranous/cytoplasmic staining. Adsorption with recombinant CYP2D6 substantially reduced plasma membrane staining and LKM1 immunoblot reactivity. Plasma membrane staining of LKM1 colocalised with that of anti-CYP2D6. Immunoprecipitation experiments showed that a single 50 kDa protein recognised by anti-CYP2D6 can be isolated from the plasma membrane of intact hepatocytes. CONCLUSIONS—AIH and HCV related LKM1 recognise CYP2D6 exposed on the plasma membrane of isolated hepatocytes. This observation supports the notion that anti-CYP2D6 autoreactivity may be involved in the pathogenesis of liver damage. Keywords: liver/kidney microsomal antibody type 1; autoimmunity; autoimmune hepatitis; hepatitis C virus infection; confocal microscopy
Full Text
The Full Text of this article is available as a PDF (228.6 KB).
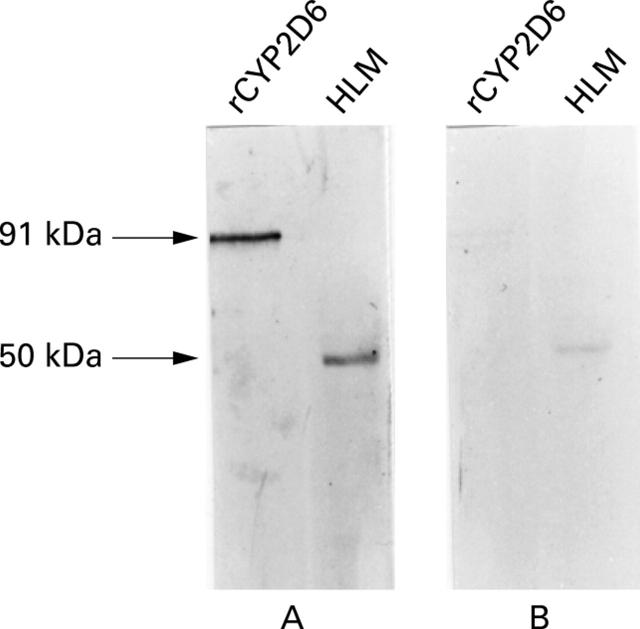
Figure 1 .
Immunoblotting reactivity of a representative liver/kidney microsomal antibody type 1 (LKM1) positive serum with recombinant human CYP2D6 (rCYP2D6) and human liver microsomes (HLM). Serum reacts with a 91 kDa polypeptide, corresponding to rCYP2D6 expressed in Escherichia coli and with a 50 kDa polypeptide corresponding to native human CYP2D6 (A). After preadsorption with rCYP2D6, the reactivity with rCYP2D6 is completely lost, whereas a faint 50 kDa band is still visible with native CYP2D6 (B).
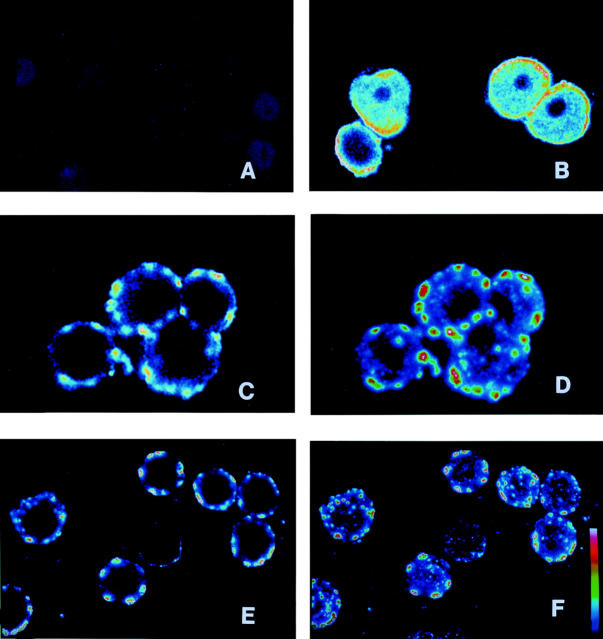
Figure 2 .
Confocal microscope images of plasma membrane staining by liver/kidney microsomal antibody type 1 (LKM1) positive sera in freshly isolated rat hepatocytes. The hepatocytes were probed with LKM1 positive sera (diluted 1:50) and fluorescein labelled goat anti-human IgG (diluted 1:200). The images correspond to: (A) non-permeabilised hepatocytes exposed to the serum of a healthy control; (B) permeabilised hepatocytes probed with the serum of a LKM1 positive subject; (C-F) non-permeabilised hepatocytes probed with LKM1 positive sera from a patient with AIH (C,D) or a patient with chronic HCV infection (E,F). Images D and F were obtained by merging nine focal frames taken along the z axis at 1 µm intervals. Images B, C, and E show the focal frame corresponding to the median optical section of the hepatocytes. Fluorescence is shown in pseudocolours increasing from blue to red and white. Original magnifications are 30 × for images A, B, E, and F and 60 × for images C and D.
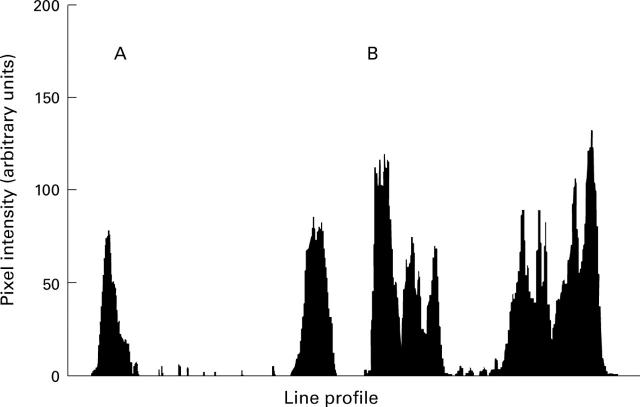
Figure 3 .
Frequency distribution of the pixels referring to cellular fluorescence in optical sections from hepatocytes stained with liver/kidney microsomal antibody type 1 (LKM1) positive serum (diluted 1:40) and fluorescein labelled goat anti-human IgG (diluted 1:200). (A) Typical distribution in non-permeabilised hepatocytes; (B) distribution in hepatocytes that were permeabilised before fixation as reported in the Methods section.
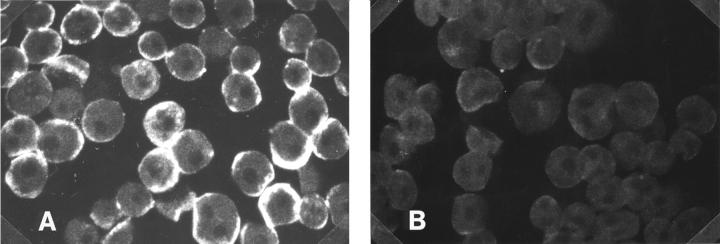
Figure 4 .
Effect of the preadsorption of liver/kidney microsomal antibody type 1 (LKM1) positive sera with recombinant human CYP2D6 on the surface staining of isolated rat hepatocytes. LKM1 sera were preadsorbed as described in the Methods section with human recombinant CYP2D6. Non-permeabilised rat hepatocytes were stained with native (A) and preadsorbed (B) sera at the same dilution (1:200). Original magnification × 10.
Figure 5 .
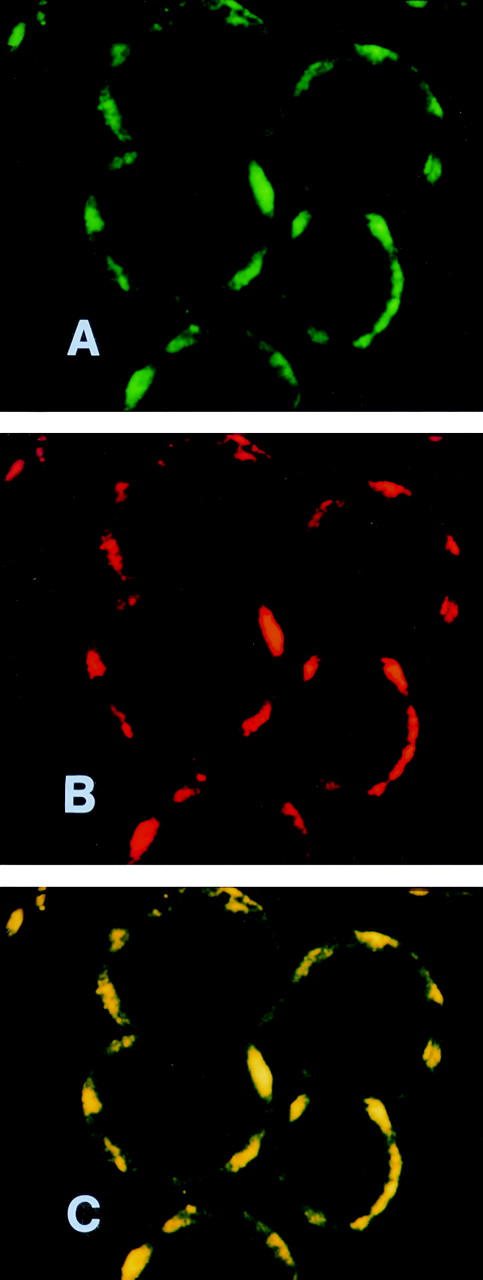
Confocal microscope images of the same focal section of adjacent rat hepatocytes allowed to react with rabbit anti-CYP2D6 serum and liver/kidney microsomal antibody type 1 (LKM1) positive human serum. The cell preparation was double stained with goat anti-human IgG labelled with fluorescein and with goat anti-rabbit IgG complexed with Texas Red. The same median cell optical section shows the green fluorescence due to fluorescein in (A) and the red fluorescence of Texas Red in (B). (C) Result of the electronic merging of images (A) and (B). Original magnification × 30.
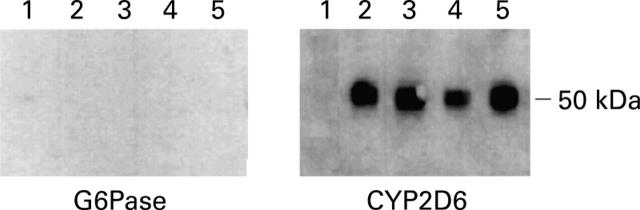
Figure 6 .
Immunoprecipitation of antigens recognised by human liver/kidney microsomal antibody type 1 (LKM1) sera on hepatocyte plasma membranes. Plasma membrane antigens of isolated hepatocytes were immunoprecipitated by using human LKM1 positive sera from either autoimmune or HCV related hepatitis patients complexed with Protein A coated Sepharose beads. The precipitated proteins were submitted to SDS/PAGE and visualised using a rabbit polyclonal anti-human CYP2D6 serum. Lane 1, control beads without attached LKM1 serum; lanes 2 and 3, two different LKM1 positive sera from patients with autoimmune hepatitis; lanes 4 and 5, two different LKM1 positive sera from patients with hepatitis C virus (HCV) hepatitis. G6Pase, glucose-6-phosphatase.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaune P., Pessayre D., Dansette P., Mansuy D., Manns M. Autoantibodies against cytochromes P450: role in human diseases. Adv Pharmacol. 1994;30:199–245. doi: 10.1016/s1054-3589(08)60175-1. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Fairfax A., Swana G., Doniach D., Groeschel-Stewart U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol. 1976 May;29(5):403–410. doi: 10.1136/jcp.29.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani F., Cataleta M., Valentini P., Muratori P., Giostra F., Francesconi R., Muratori L., Lenzi M., Bianchi G., Zauli D. Serum autoantibodies in chronic hepatitis C: comparison with autoimmune hepatitis and impact on the disease profile. Hepatology. 1997 Sep;26(3):561–566. doi: 10.1002/hep.510260305. [DOI] [PubMed] [Google Scholar]
- Choudhuri K., Mieli-Vergani G., Vergani D. Cytochrome P4502D6: understanding an autoantigen. Clin Exp Immunol. 1997 Jun;108(3):381–383. doi: 10.1046/j.1365-2249.1997.4131311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clot P., Parola M., Bellomo G., Dianzani U., Carini R., Tabone M., Aricò S., Ingelman-Sundberg M., Albano E. Plasma membrane hydroxyethyl radical adducts cause antibody-dependent cytotoxicity in rat hepatocytes exposed to alcohol. Gastroenterology. 1997 Jul;113(1):265–276. doi: 10.1016/s0016-5085(97)70104-5. [DOI] [PubMed] [Google Scholar]
- Eliasson E., Kenna J. G. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996 Sep;50(3):573–582. [PubMed] [Google Scholar]
- Gregorio G. V., Portmann B., Reid F., Donaldson P. T., Doherty D. G., McCartney M., Mowat A. P., Vergani D., Mieli-Vergani G. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology. 1997 Mar;25(3):541–547. doi: 10.1002/hep.510250308. [DOI] [PubMed] [Google Scholar]
- Homberg J. C., Abuaf N., Bernard O., Islam S., Alvarez F., Khalil S. H., Poupon R., Darnis F., Lévy V. G., Grippon P. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of "autoimmune" hepatitis. Hepatology. 1987 Nov-Dec;7(6):1333–1339. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., McFarlane I. G. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993 Oct;18(4):998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- Krohn K., Uibo R., Aavik E., Peterson P., Savilahti K. Identification by molecular cloning of an autoantigen associated with Addison's disease as steroid 17 alpha-hydroxylase. Lancet. 1992 Mar 28;339(8796):770–773. doi: 10.1016/0140-6736(92)91894-e. [DOI] [PubMed] [Google Scholar]
- Leeder J. S., Gaedigk A., Lu X., Cook V. A. Epitope mapping studies with human anti-cytochrome P450 3A antibodies. Mol Pharmacol. 1996 Feb;49(2):234–243. [PubMed] [Google Scholar]
- Lenzi M., Bianchi F. B., Cassani F., Pisi E. Liver cell surface expression of the antigen reacting with liver-kidney microsomal antibody (LKM). Clin Exp Immunol. 1984 Jan;55(1):36–40. [PMC free article] [PubMed] [Google Scholar]
- Lenzi M., Fusconi M., Selleri L., Caselli A., Cassani F., Bianchi F. B., Pisi E. Counterimmunoelectrophoresis (CIE) for the detection of anti-liver-kidney microsome (LKM) antibodies in the sera of patients with chronic liver disease. J Immunol Methods. 1988 Jul 22;111(2):253–259. doi: 10.1016/0022-1759(88)90134-2. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Eddershaw P. J., Goldfarb P. S., Tarbit M. H. Molecular modelling of cytochrome P4502D6 (CYP2D6) based on an alignment with CYP102: structural studies on specific CYP2D6 substrate metabolism. Xenobiotica. 1997 Apr;27(4):319–339. doi: 10.1080/004982597240497. [DOI] [PubMed] [Google Scholar]
- Loeper J., Descatoire V., Maurice M., Beaune P., Belghiti J., Houssin D., Ballet F., Feldmann G., Guengerich F. P., Pessayre D. Cytochromes P-450 in human hepatocyte plasma membrane: recognition by several autoantibodies. Gastroenterology. 1993 Jan;104(1):203–216. doi: 10.1016/0016-5085(93)90853-5. [DOI] [PubMed] [Google Scholar]
- Loeper J., Descatoire V., Maurice M., Beaune P., Feldmann G., Larrey D., Pessayre D. Presence of functional cytochrome P-450 on isolated rat hepatocyte plasma membrane. Hepatology. 1990 May;11(5):850–858. doi: 10.1002/hep.1840110521. [DOI] [PubMed] [Google Scholar]
- Loeper J., Louérat-Oriou B., Duport C., Pompon D. Yeast expressed cytochrome P450 2D6 (CYP2D6) exposed on the external face of plasma membrane is functionally competent. Mol Pharmacol. 1998 Jul;54(1):8–13. doi: 10.1124/mol.54.1.8. [DOI] [PubMed] [Google Scholar]
- Lytton S. D., Helander A., Zhang-Gouillon Z. Q., Stokkeland K., Bordone R., Aricò S., Albano E., French S. W., Ingelman-Sundberg M. Autoantibodies against cytochromes P-4502E1 and P-4503A in alcoholics. Mol Pharmacol. 1999 Feb;55(2):223–233. doi: 10.1124/mol.55.2.223. [DOI] [PubMed] [Google Scholar]
- Löhr H. F., Schlaak J. F., Lohse A. W., Böcher W. O., Arenz M., Gerken G., Meyer Zum Büschenfelde K. H. Autoreactive CD4+ LKM-specific and anticlonotypic T-cell responses in LKM-1 antibody-positive autoimmune hepatitis. Hepatology. 1996 Dec;24(6):1416–1421. doi: 10.1002/hep.510240619. [DOI] [PubMed] [Google Scholar]
- Löhr H., Manns M., Kyriatsoulis A., Lohse A. W., Trautwein C., Meyer zum Büschenfelde K. H., Fleischer B. Clonal analysis of liver-infiltrating T cells in patients with LKM-1 antibody-positive autoimmune chronic active hepatitis. Clin Exp Immunol. 1991 May;84(2):297–302. doi: 10.1111/j.1365-2249.1991.tb08164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Gregorio G., Gäken J., Muratori L., Bianchi F. B., Mieli-Vergani G., Vergani D. Establishment of a novel radioligand assay using eukaryotically expressed cytochrome P4502D6 for the measurement of liver kidney microsomal type 1 antibody in patients with autoimmune hepatitis and hepatitis C virus infection. J Hepatol. 1997 Jun;26(6):1396–1402. doi: 10.1016/s0168-8278(97)80477-1. [DOI] [PubMed] [Google Scholar]
- Ma Y., Peakman M., Lobo-Yeo A., Wen L., Lenzi M., Gäken J., Farzaneh F., Mieli-Vergani G., Bianchi F. B., Vergani D. Differences in immune recognition of cytochrome P4502D6 by liver kidney microsomal (LKM) antibody in autoimmune hepatitis and chronic hepatitis C virus infection. Clin Exp Immunol. 1994 Jul;97(1):94–99. doi: 10.1111/j.1365-2249.1994.tb06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie F. D., Peakman M., Yun M., Sallie R., Smith H., Davies E. T., Mieli-Vergani G., Vergani D. Primary and secondary liver/kidney microsomal autoantibody response following infection with hepatitis C virus. Gastroenterology. 1994 Jun;106(6):1672–1675. doi: 10.1016/0016-5085(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Manns M. P., Johnson E. F., Griffin K. J., Tan E. M., Sullivan K. F. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450db1. J Clin Invest. 1989 Mar;83(3):1066–1072. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini E., Abuaf N., Cavalli F., Durand V., Johanet C., Homberg J. C. Antibody to liver cytosol (anti-LC1) in patients with autoimmune chronic active hepatitis type 2. Hepatology. 1988 Nov-Dec;8(6):1662–1666. doi: 10.1002/hep.1840080632. [DOI] [PubMed] [Google Scholar]
- Maslansky C. J., Williams G. M. Primary cultures and the levels of cytochrome P450 in hepatocytes from mouse, rat, hamster, and rabbit liver. In Vitro. 1982 Aug;18(8):683–693. doi: 10.1007/BF02796423. [DOI] [PubMed] [Google Scholar]
- Mieli-Vergani G., Vergani D. Progress in pediatric autoimmune hepatitis. Semin Liver Dis. 1994 Aug;14(3):282–288. doi: 10.1055/s-2007-1007318. [DOI] [PubMed] [Google Scholar]
- Muratori L., Lenzi M., Ma Y., Cataleta M., Mieli-Vergani G., Vergani D., Bianchi F. B. Heterogeneity of liver/kidney microsomal antibody type 1 in autoimmune hepatitis and hepatitis C virus related liver disease. Gut. 1995 Sep;37(3):406–412. doi: 10.1136/gut.37.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratori L., Zauli D., Giostra F., Ballardini G., Lenzi M., Cassani F., Bianchi F. B. LKM1 appearance in a HLA-DR3+ patient with chronic hepatitis C during interferon treatment. J Hepatol. 1993 Jun;18(2):259–260. doi: 10.1016/s0168-8278(05)80258-2. [DOI] [PubMed] [Google Scholar]
- Myrset A. H., Halvorsen B., Ording E., Helgeland L. The time courses of intracellular transport of some secretory proteins of rat liver are not affected by an induced acute phase response. Eur J Cell Biol. 1993 Feb;60(1):108–114. [PubMed] [Google Scholar]
- Neve E. P., Eliasson E., Pronzato M. A., Albano E., Marinari U., Ingelman-Sundberg M. Enzyme-specific transport of rat liver cytochrome P450 to the Golgi apparatus. Arch Biochem Biophys. 1996 Sep 15;333(2):459–465. doi: 10.1006/abbi.1996.0415. [DOI] [PubMed] [Google Scholar]
- Pessayre D. Role of reactive metabolites in drug-induced hepatitis. J Hepatol. 1995;23 (Suppl 1):16–24. [PubMed] [Google Scholar]
- Riley R. J., Smith G., Wolf C. R., Cook V. A., Leeder J. S. Human anti-endoplasmic reticulum autoantibodies produced in aromatic anticonvulsant hypersensitivity reactions recognise rodent CYP3A proteins and a similarly regulated human P450 enzyme(s) Biochem Biophys Res Commun. 1993 Feb 26;191(1):32–40. doi: 10.1006/bbrc.1993.1180. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Swana G., Doniach D. Microsomal antibodies in active chronic hepatitis and other disorders. Clin Exp Immunol. 1973 Nov;15(3):331–344. [PMC free article] [PubMed] [Google Scholar]
- Robin M. A., Le Roy M., Descatoire V., Pessayre D. Plasma membrane cytochromes P450 as neoantigens and autoimmune targets in drug-induced hepatitis. J Hepatol. 1997;26 (Suppl 1):23–30. doi: 10.1016/s0168-8278(97)82329-x. [DOI] [PubMed] [Google Scholar]
- Robin M. A., Maratrat M., Le Roy M., Le Breton F. P., Bonierbale E., Dansette P., Ballet F., Mansuy D., Pessayre D. Antigenic targets in tienilic acid hepatitis. Both cytochrome P450 2C11 and 2C11-tienilic acid adducts are transported to the plasma membrane of rat hepatocytes and recognized by human sera. J Clin Invest. 1996 Sep 15;98(6):1471–1480. doi: 10.1172/JCI118936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin M. A., Maratrat M., Loeper J., Durand-Schneider A. M., Tinel M., Ballet F., Beaune P., Feldmann G., Pessayre D. Cytochrome P4502B follows a vesicular route to the plasma membrane in cultured rat hepatocytes. Gastroenterology. 1995 Apr;108(4):1110–1123. doi: 10.1016/0016-5085(95)90210-4. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Pitot H. C. Drug metabolism and effects of carcinogens in cultured hepatic cells. Pharmacol Rev. 1979 Sep;31(3):205–228. [PubMed] [Google Scholar]
- Trautwein C., Gerken G., Löhr H., Meyer zum Büschenfelde K. H., Manns M. Lack of surface expression for the B-cell autoepitope of cytochrome P450 IID6 evidenced by flow cytometry. Z Gastroenterol. 1993 Apr;31(4):225–230. [PubMed] [Google Scholar]
- Van Pelt F. N., Straub P., Manns M. P. Molecular basis of drug-induced immunological liver injury. Semin Liver Dis. 1995 Aug;15(3):283–300. doi: 10.1055/s-2007-1007281. [DOI] [PubMed] [Google Scholar]
- Vergani D., Mieli-Vergani G. Type II autoimmune hepatitis: the conundrum of cytochrome P450IID6. Clin Exp Immunol. 1993 Jun;92(3):367–368. doi: 10.1111/j.1365-2249.1993.tb03406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winqvist O., Gustafsson J., Rorsman F., Karlsson F. A., Kämpe O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison's disease. J Clin Invest. 1993 Nov;92(5):2377–2385. doi: 10.1172/JCI116843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winqvist O., Karlsson F. A., Kämpe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison's disease. Lancet. 1992 Jun 27;339(8809):1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- Wu D., Cederbaum A. I. Presence of functionally active cytochrome P-450IIE1 in the plasma membrane of rat hepatocytes. Hepatology. 1992 Mar;15(3):515–524. doi: 10.1002/hep.1840150326. [DOI] [PubMed] [Google Scholar]
- Yamamoto A. M., Cresteil D., Homberg J. C., Alvarez F. Characterization of anti-liver-kidney microsome antibody (anti-LKM1) from hepatitis C virus-positive and -negative sera. Gastroenterology. 1993 Jun;104(6):1762–1767. doi: 10.1016/0016-5085(93)90657-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto A. M., Johanet C., Duclos-Vallee J. C., Bustarret F. A., Alvarez F., Homberg J. C., Bach J. F. A new approach to cytochrome CYP2D6 antibody detection in autoimmune hepatitis type-2 (AIH-2) and chronic hepatitis C virus (HCV) infection: a sensitive and quantitative radioligand assay. Clin Exp Immunol. 1997 Jun;108(3):396–400. doi: 10.1046/j.1365-2249.1997.4071302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A. M., Mura C., De Lemos-Chiarandini C., Krishnamoorthy R., Alvarez F. Cytochrome P450IID6 recognized by LKM1 antibody is not exposed on the surface of hepatocytes. Clin Exp Immunol. 1993 Jun;92(3):381–390. doi: 10.1111/j.1365-2249.1993.tb03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger U. M., Hauri H. P., Loeper J., Homberg J. C., Meyer U. A. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8256–8260. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]