Abstract
BACKGROUND—In patients with Peutz-Jeghers syndrome (PJS), causative germline mutations in the LKB1/STK11 gene on chromosome 19p13.3 have been identified. Because of the loss of heterozygosity (LOH) at 19p13.3 in hamartomas and the cancer susceptibility of patients with PJS, LKB1/STK11 is suggested to act as a tumour suppressor. However, the frequency of genetic and epigenetic inactivation of LKB1/STK11 in sporadic tumours is unclear. AIMS—To investigate the LKB1/STK11 gene for promoter hypermethylation and allelic loss in tumour specimens of patients with sporadic colorectal cancer. METHODS—DNA from 50 consecutive paraffin embedded sporadic colorectal adenocarcinomas and corresponding normal epithelium was extracted. After bisulphite treatment, specimens were analysed for methylation of the LKB1/STK11 promoter 5'-CpG island by methylation specific polymerase chain reaction (MSP). In addition, tumours were analysed for LOH of chromosome 19p13.3. In tumours exhibiting LOH, LKB1/STK11 was sequenced. RESULTS—MSP was successful in 48 of 50 tumour specimens. Of those, four (8%) demonstrated hypermethylation of the LKB1/STK11 promoter 5'-CpG island. Moreover, LOH at either D19S886 or D19S878 was observed in five of 38 (13%) informative tumours. All five tumours showing LOH at 19p13.3 were advanced and four of five were located in the left sided colon. There was no correlation between LOH and LKB1/STK11 promoter hypermethylation or somatic mutation. CONCLUSIONS—In sporadic colorectal cancer, hypermethylation of the LKB1/STK11 promoter and allelic loss at the STK 11 gene locus are rare events. LOH at 19p13.3 was associated with advanced tumour stage and left sided location but not with LKB1/STK11 promoter hypermethylation or somatic mutation. Keywords: sporadic colorectal adenocarcinoma; tumour suppressor genes; protein-serine-threonine kinase gene LKB1/STK11; promoter hypermethylation; loss of heterozygosity
Full Text
The Full Text of this article is available as a PDF (122.1 KB).
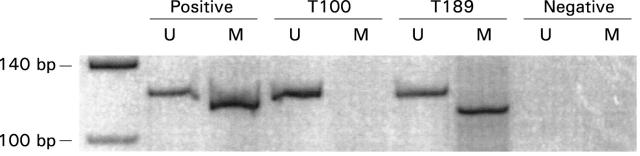
Figure 1 .
Methylation specific polymerase chain reaction (PCR) of the LKB1/STK11 promoter 5'-CpG island in sporadic colorectal cancer. In the left lane the 140 bp and 100 bp bands of a molecular weight standard are shown. The presence of a visible PCR product in those lanes marked U indicates unmethylated LKB1/STK11 promoter 5'-CpG islands; a visible PCR product in those lanes marked M indicates the presence of methylated LKB1/STK11 promoter 5'-CpG islands. Corresponding lanes are: positive controls for PCR reactions (for the unmethylated reaction DNA extracted from the colorectal cancer cell line HT29 and for the methylated reaction from the colorectal cancer cell line H6), primary colorectal cancers (T100 and T189), and negative controls for PCR reactions.
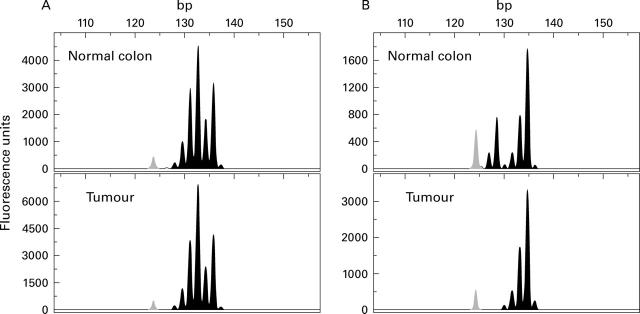
Figure 2 .
Fluorescent analysis of the microsatellite marker D19S878 (centromeric to the LKB1/STK11 gene locus) on chromosome 19p13.3. The 123 bp peak of the size standard is plotted in light grey in all electrophoretic profiles. The polymerase chain reaction (PCR) products of normal colon tissue (upper panels) and corresponding tumour tissue (lower panels) in patient No 31 (A) and No 3 (B) with sporadic colorectal adenocarcinoma were electrophoretically analysed on an automated ABI 310 DNA sequencer (Perkin Elmer). (A) Tumour without allelic loss at D19S878. (B) Tumour exhibiting loss of the 127 bp allele of D19S878 whereas the 134 bp allele is conserved.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos C. I., Bali D., Thiel T. J., Anderson J. P., Gourley I., Frazier M. L., Lynch P. M., Luchtefeld M. A., Young A., McGarrity T. J. Fine mapping of a genetic locus for Peutz-Jeghers syndrome on chromosome 19p. Cancer Res. 1997 Sep 1;57(17):3653–3656. [PubMed] [Google Scholar]
- Avizienyte E., Loukola A., Roth S., Hemminki A., Tarkkanen M., Salovaara R., Arola J., Bützow R., Husgafvel-Pursiainen K., Kokkola A. LKB1 somatic mutations in sporadic tumors. Am J Pathol. 1999 Mar;154(3):677–681. doi: 10.1016/S0002-9440(10)65314-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avizienyte E., Roth S., Loukola A., Hemminki A., Lothe R. A., Stenwig A. E., Fosså S. D., Salovaara R., Aaltonen L. A. Somatic mutations in LKB1 are rare in sporadic colorectal and testicular tumors. Cancer Res. 1998 May 15;58(10):2087–2090. [PubMed] [Google Scholar]
- Bignell G. R., Barfoot R., Seal S., Collins N., Warren W., Stratton M. R. Low frequency of somatic mutations in the LKB1/Peutz-Jeghers syndrome gene in sporadic breast cancer. Cancer Res. 1998 Apr 1;58(7):1384–1386. [PubMed] [Google Scholar]
- Boardman L. A., Thibodeau S. N., Schaid D. J., Lindor N. M., McDonnell S. K., Burgart L. J., Ahlquist D. A., Podratz K. C., Pittelkow M., Hartmann L. C. Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann Intern Med. 1998 Jun 1;128(11):896–899. doi: 10.7326/0003-4819-128-11-199806010-00004. [DOI] [PubMed] [Google Scholar]
- Dong S. M., Kim K. M., Kim S. Y., Shin M. S., Na E. Y., Lee S. H., Park W. S., Yoo N. J., Jang J. J., Yoon C. Y. Frequent somatic mutations in serine/threonine kinase 11/Peutz-Jeghers syndrome gene in left-sided colon cancer. Cancer Res. 1998 Sep 1;58(17):3787–3790. [PubMed] [Google Scholar]
- Esteller M., Avizienyte E., Corn P. G., Lothe R. A., Baylin S. B., Aaltonen L. A., Herman J. G. Epigenetic inactivation of LKB1 in primary tumors associated with the Peutz-Jeghers syndrome. Oncogene. 2000 Jan 6;19(1):164–168. doi: 10.1038/sj.onc.1203227. [DOI] [PubMed] [Google Scholar]
- Esteller M., Hamilton S. R., Burger P. C., Baylin S. B., Herman J. G. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999 Feb 15;59(4):793–797. [PubMed] [Google Scholar]
- Giardiello F. M., Welsh S. B., Hamilton S. R., Offerhaus G. J., Gittelsohn A. M., Booker S. V., Krush A. J., Yardley J. H., Luk G. D. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987 Jun 11;316(24):1511–1514. doi: 10.1056/NEJM198706113162404. [DOI] [PubMed] [Google Scholar]
- Gruber S. B., Entius M. M., Petersen G. M., Laken S. J., Longo P. A., Boyer R., Levin A. M., Mujumdar U. J., Trent J. M., Kinzler K. W. Pathogenesis of adenocarcinoma in Peutz-Jeghers syndrome. Cancer Res. 1998 Dec 1;58(23):5267–5270. [PubMed] [Google Scholar]
- Guldberg P., thor Straten P., Ahrenkiel V., Seremet T., Kirkin A. F., Zeuthen J. Somatic mutation of the Peutz-Jeghers syndrome gene, LKB1/STK11, in malignant melanoma. Oncogene. 1999 Mar 4;18(9):1777–1780. doi: 10.1038/sj.onc.1202486. [DOI] [PubMed] [Google Scholar]
- Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Höglund P. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998 Jan 8;391(6663):184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Hemminki A., Tomlinson I., Markie D., Järvinen H., Sistonen P., Björkqvist A. M., Knuutila S., Salovaara R., Bodmer W., Shibata D. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet. 1997 Jan;15(1):87–90. doi: 10.1038/ng0197-87. [DOI] [PubMed] [Google Scholar]
- Herman J. G. Hypermethylation of tumor suppressor genes in cancer. Semin Cancer Biol. 1999 Oct;9(5):359–367. doi: 10.1006/scbi.1999.0138. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Reimann H., Nezu J., Friedel W., Loff S., Jeschke R., Müller O., Back W., Zimmer M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998 Jan;18(1):38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Laird P. W. Cancer epigenetics comes of age. Nat Genet. 1999 Feb;21(2):163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996 Oct 18;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Knudson A. G. Antioncogenes and human cancer. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch H. T., Lynch J. F. Genetics of colonic cancer. Digestion. 1998 Aug;59(5):481–492. doi: 10.1159/000007525. [DOI] [PubMed] [Google Scholar]
- Mehenni H., Gehrig C., Nezu J., Oku A., Shimane M., Rossier C., Guex N., Blouin J. L., Scott H. S., Antonarakis S. E. Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am J Hum Genet. 1998 Dec;63(6):1641–1650. doi: 10.1086/302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W. S., Moon Y. W., Yang Y. M., Kim Y. S., Kim Y. D., Fuller B. G., Vortmeyer A. O., Fogt F., Lubensky I. A., Zhuang Z. Mutations of the STK11 gene in sporadic gastric carcinoma. Int J Oncol. 1998 Sep;13(3):601–604. [PubMed] [Google Scholar]
- Resta N., Simone C., Mareni C., Montera M., Gentile M., Susca F., Gristina R., Pozzi S., Bertario L., Bufo P. STK11 mutations in Peutz-Jeghers syndrome and sporadic colon cancer. Cancer Res. 1998 Nov 1;58(21):4799–4801. [PubMed] [Google Scholar]
- Rowan A., Bataille V., MacKie R., Healy E., Bicknell D., Bodmer W., Tomlinson I. Somatic mutations in the Peutz-Jeghers (LKB1/STKII) gene in sporadic malignant melanomas. J Invest Dermatol. 1999 Apr;112(4):509–511. doi: 10.1046/j.1523-1747.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- Spigelman A. D., Murday V., Phillips R. K. Cancer and the Peutz-Jeghers syndrome. Gut. 1989 Nov;30(11):1588–1590. doi: 10.1136/gut.30.11.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G. H., Hruban R. H., Bansal R. K., Bova G. S., Tang D. J., Shekher M. C., Westerman A. M., Entius M. M., Goggins M., Yeo C. J. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999 Jun;154(6):1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. Y., Erikson E., Maller J. L. Cloning and characterization of a novel serine/threonine protein kinase expressed in early Xenopus embryos. J Biol Chem. 1996 Jun 14;271(24):14430–14437. doi: 10.1074/jbc.271.24.14430. [DOI] [PubMed] [Google Scholar]
- Trojan J., Brieger A., Raedle J., Roth W. K., Zeuzem S. Peutz-Jeghers syndrome: molecular analysis of a three-generation kindred with a novel defect in the serine threonine kinase gene STK11. Am J Gastroenterol. 1999 Jan;94(1):257–261. doi: 10.1111/j.1572-0241.1999.00810.x. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Wang Z. J., Churchman M., Campbell I. G., Xu W. H., Yan Z. Y., McCluggage W. G., Foulkes W. D., Tomlinson I. P. Allele loss and mutation screen at the Peutz-Jeghers (LKB1) locus (19p13.3) in sporadic ovarian tumours. Br J Cancer. 1999 Apr;80(1-2):70–72. doi: 10.1038/sj.bjc.6690323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. J., Taylor F., Churchman M., Norbury G., Tomlinson I. Genetic pathways of colorectal carcinogenesis rarely involve the PTEN and LKB1 genes outside the inherited hamartoma syndromes. Am J Pathol. 1998 Aug;153(2):363–366. doi: 10.1016/S0002-9440(10)65579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikorkala A., Avizienyte E., Tomlinson I. P., Tiainen M., Roth S., Loukola A., Hemminki A., Johansson M., Sistonen P., Markie D. Mutations and impaired function of LKB1 in familial and non-familial Peutz-Jeghers syndrome and a sporadic testicular cancer. Hum Mol Genet. 1999 Jan;8(1):45–51. doi: 10.1093/hmg/8.1.45. [DOI] [PubMed] [Google Scholar]