Abstract
BACKGROUND—In the intestinal mucosa, numerous cytokines produced by the epithelium, fibroblasts, and immune cells were shown to affect epithelial differentiation and proliferation through epithelial-mesenchymal and epithelial-immune cell interactions. To date, the importance of cytokines in postnatal development of the rat small intestine has not been established. AIM—To investigate the developmental changes in expression of mucosal cytokines in the postnatal maturation of the rat small intestinal epithelium and their regulation by glucocorticoids (GC). METHODS—Mucosal maturation was assessed by the onset of sucrase-isomaltase (SI) mRNA, analysed by in situ hybridisation. The amount of transforming growth factor β1 (TGF-β1), β2 (TGF-β2), tumour necrosis factor α (TNF-α), interleukin 1β (IL-1β), and TGF-α was analysed by reverse transcription-polymerase chain reaction (RT-PCR) in mucosal extracts from weaning (14-21 days old) and adult rats, or one day after an injection of hydrocortisone (HC) in 11 day old rats. Similarly, expression of cytokines and the regulatory effect of GC were studied on cultured subepithelial myofibroblasts cloned from postnatal jejunum and ileum cultured in the absence or presence of dexamethasone (DX). RESULTS—TGF-β1, TGF-β2, and IL-1β decreased during the third week of life while levels of TNF-α increased and TGF-α remained constant. In parallel, SI transcripts increased and showed a progressive accumulation in the apical part of the enterocytes first localised at the base of the villi from 18 days onwards. Interestingly, precocious induction of SI mRNA by HC paralleled the decrease in expression of TGF-β isoforms and of IL-1β. All cytokines were expressed in the myofibroblast cell lines. In addition, the results showed that TNF-α was differentially expressed in basal culture conditions and after DX stimulation in jejunal and ileal myofibroblasts. DX decreased IL-1β but not the TGF-β isoforms, similar to that in vivo. CONCLUSIONS—This study shows that mucosal cytokines are developmentally regulated and that GC are potentially involved in this regulation in parallel with maturation of the gut mucosa at weaning. Keywords: small intestine; weaning; maturation; fibroblasts; cytokines
Full Text
The Full Text of this article is available as a PDF (244.2 KB).
Figure 1 .
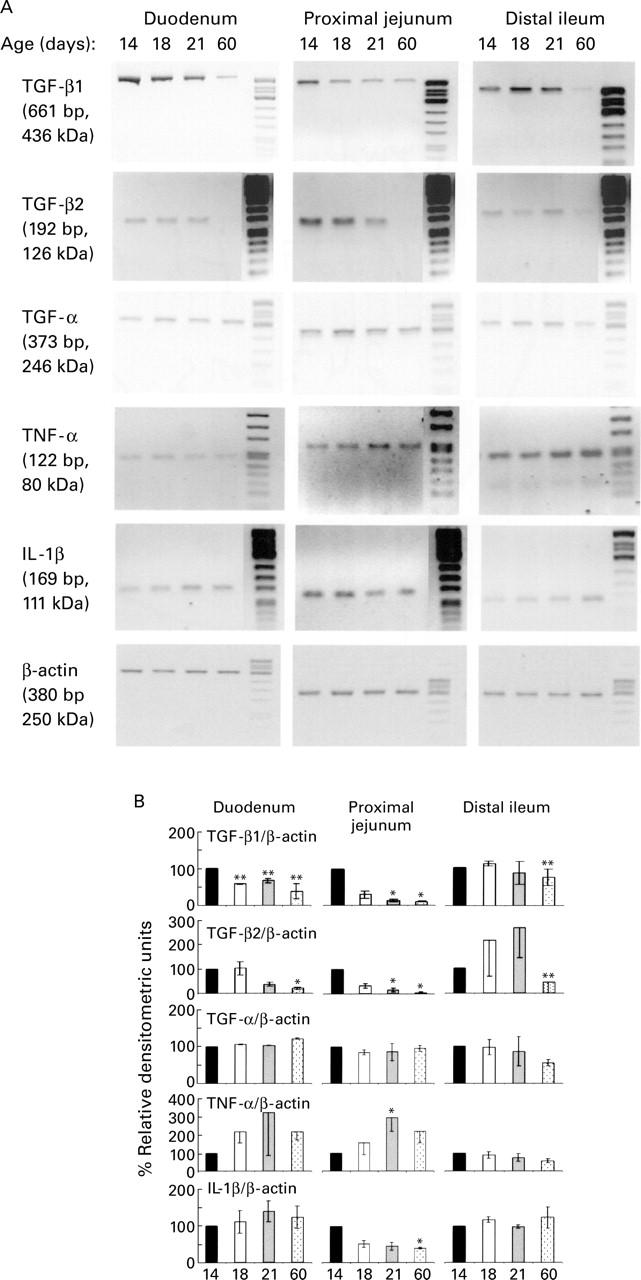
Representative illustration of the developmental pattern of cytokine expression in the small intestinal mucosa from suckling rats. (A) RT-PCR analysis of transforming growth factor β1 and β2 (TGF-β1, TGF-β2), transforming growth factor α (TGF-α), tumour necrosis factor α (TNF-α), and interleukin 1β (IL-1β) in the duodenum, proximal jejunum, and distal ileum of 14, 18, 21, and 60 day old rats. (B) Densitometric analysis of the specific bands normalised to the corresponding values of β-actin mRNA used as internal control; mean (SEM) of values (expressed as percentages of the relative intensity of the bands at 14 days) obtained from three independent experiments. Significant differences compared with values obtained in 14 day old suckling rats (unpaired t test): *p<0.05, **p<0.01.
Figure 2 .
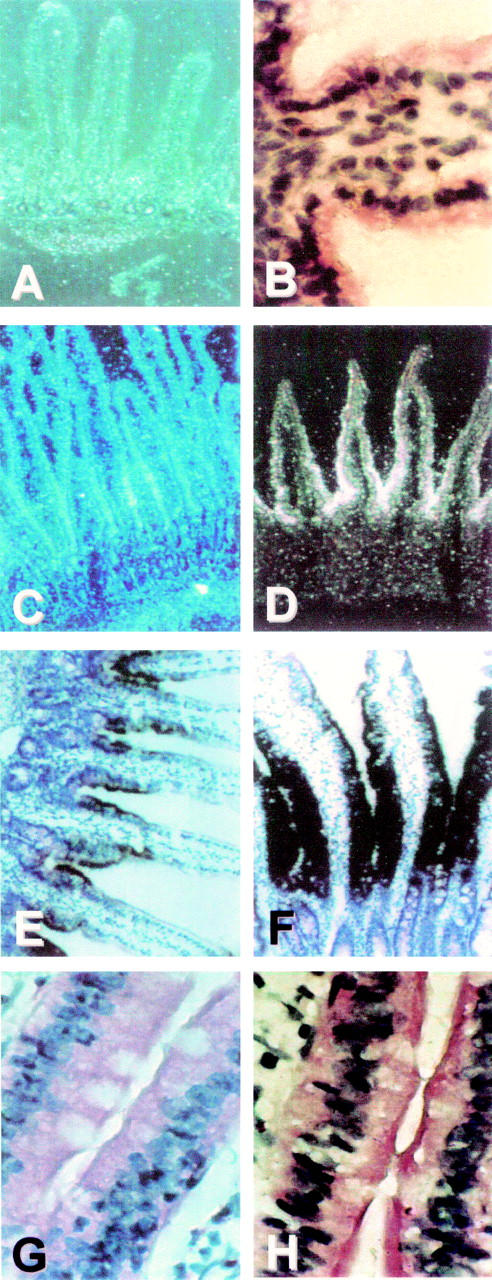
Developmental pattern of sucrase-isomaltase (SI) mRNA expression. In situ hybridisation was performed on sections of the proximal jejunum using an SI antisense cRNA probe as described in materials and methods. Dark field (A, C, D) and bright field illuminations (B, E-H) of intestinal sections from 11 (A, B), 16 (C), 18 (D), 21 (E), and 60 (F-H) day old rats. Controls: hybridisation with a sense probe (G) or treatment of the section with RNAse before hybridisation (H). Magnifications: A, C-F ×50; B, G, H, ×250.
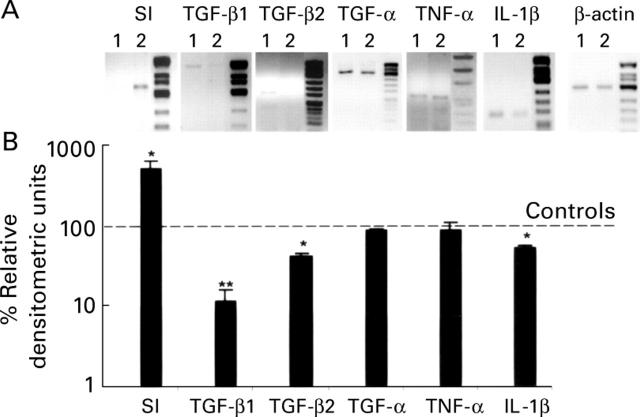
Figure 3 .
Effects of hydrocortisone (HC), injected in 11 day old pups, on sucrase-isomaltase (SI) induction and cytokine expression. (A) Representative RT-PCR assays detecting SI, transforming growth factor β1 and β2 (TGF-β1, TGF-β2), transforming growth factor α (TGF-α), tumour necrosis factor α (TNF-α), interleukin 1β (IL-1β), and β-actin mRNAs at postnatal day 12: lanes 1, control; lanes 2, treated animals. (B) Densitometric analysis of SI, TGF-β1, TGF-β2, TGF-α, TNF-α, and IL-1β bands normalised to values of β-actin as internal control from three independent experiments. Results are illustrated in the HC treated tissues as percentages of control values. *p< 0.05, ** p<0.01, significant differences between HC treated and control animals (unpaired t test).
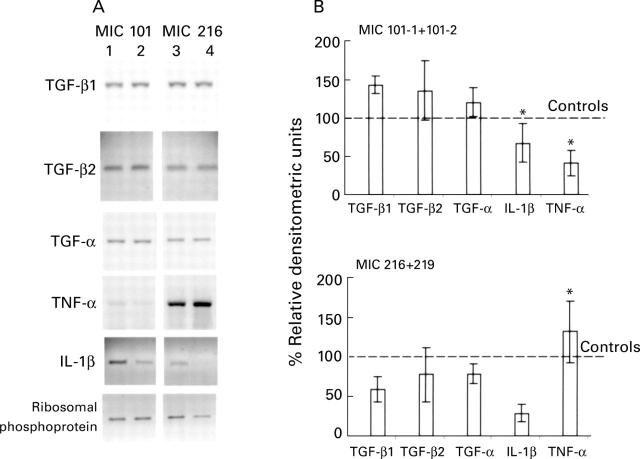
Figure 4 .
Effect of DX on expression of cytokines by subepithelial myofibroblast cell lines. (A) Representative RT-PCR analysis of transforming growth factor β1 and β2 (TGF-β1, TGF-β2), transforming growth factor α (TGF-α), tumour necrosis factor α (TNF-α), interleukin 1β (IL-1β), and ribosomal protein transcripts extracted from myofibroblasts cloned from the jejunum (MIC 101-1; lanes 1 and 2) and ileum (MIC 216; lanes 3 and 4). Myofibroblastic cells were cultured for two days in the basic medium (lanes 1 and 3) and in the presence of 8×10 -7 M DX (lanes 2 and 4). (B) Densitometric analysis of TGF-β1, TGF-β2, TGF-α, IL-1β, and TNF-α bands (normalised to values of ribosomal protein used as internal control): mean of values obtained from the experiments performed on the two jejunal (MIC 101-1 and 101-2) or the two ileal (MIC 216 and 219) cell lines which gave identical results. Results are illustrated in the DX treated cultures as percentages of the corresponding controls. *p<0.05 compared with controls (unpaired t test).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahuja S. S., Shrivastav S., Danielpour D., Balow J. E., Boumpas D. T. Regulation of transforming growth factor-beta 1 and its receptor by cyclosporine in human T lymphocytes. Transplantation. 1995 Oct 15;60(7):718–723. doi: 10.1097/00007890-199510150-00018. [DOI] [PubMed] [Google Scholar]
- Autenrieth I. B., Bucheler N., Bohn E., Heinze G., Horak I. Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with bowel inflammation. Gut. 1997 Dec;41(6):793–800. doi: 10.1136/gut.41.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyatsky M. W., Rossiter G., Podolsky D. K. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996 Apr;110(4):975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- Barnard J. A., Beauchamp R. D., Coffey R. J., Moses H. L. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beil W. J., Weller P. F., Peppercorn M. A., Galli S. J., Dvorak A. M. Ultrastructural immunogold localization of subcellular sites of TNF-alpha in colonic Crohn's disease. J Leukoc Biol. 1995 Sep;58(3):284–298. doi: 10.1002/jlb.58.3.284. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Birchmeier W. Molecular aspects of mesenchymal-epithelial interactions. Annu Rev Cell Biol. 1993;9:511–540. doi: 10.1146/annurev.cb.09.110193.002455. [DOI] [PubMed] [Google Scholar]
- Chandrasena G., Sunitha I., Lau C., Nanthakumar N. N., Henning S. J. Expression of sucrase-isomaltase mRNA along the villus-crypt axis in the rat small intestine. Cell Mol Biol. 1992 May;38(3):243–254. [PubMed] [Google Scholar]
- Cummins A. G., Labrooy J. T., Shearman D. J. The effect of cyclosporin A in delaying maturation of the small intestine during weaning in the rat. Clin Exp Immunol. 1989 Mar;75(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Dionne S., D'Agata I. D., Ruemmele F. M., Levy E., St-Louis J., Srivastava A. K., Levesque D., Seidman E. G. Tyrosine kinase and MAPK inhibition of TNF-alpha- and EGF-stimulated IEC-6 cell growth. Biochem Biophys Res Commun. 1998 Jan 6;242(1):146–150. doi: 10.1006/bbrc.1997.7922. [DOI] [PubMed] [Google Scholar]
- Dvorák B., Holubec H., LeBouton A. V., Wilson J. M., Koldovský O. Epidermal growth factor and transforming growth factor-alpha mRNA in rat small intestine: in situ hybridization study. FEBS Lett. 1994 Oct 3;352(3):291–295. doi: 10.1016/0014-5793(94)00942-2. [DOI] [PubMed] [Google Scholar]
- Dvorák B., Koldovský O. The presence of transforming growth factor-alpha in the suckling rat small intestine and pancreas and the absence in rat milk. Pediatr Res. 1994 Mar;35(3):348–353. doi: 10.1203/00006450-199403000-00015. [DOI] [PubMed] [Google Scholar]
- Eckmann L., Jung H. C., Schürer-Maly C., Panja A., Morzycka-Wroblewska E., Kagnoff M. F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993 Dec;105(6):1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- Emvo E. N., Raul F., Koch B., Neuville P., Foltzer-Jourdainne C. Sucrase-isomaltase gene expression in suckling rat intestine: hormonal, dietary, and growth factor control. J Pediatr Gastroenterol Nutr. 1996 Oct;23(3):262–269. doi: 10.1097/00005176-199610000-00010. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol. 1997 Oct;273(4 Pt 1):G769–G775. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- Fritsch C., Orian-Rousseaul V., Lefebvre O., Simon-Assmann P., Reimund J. M., Duclos B., Kedinger M. Characterization of human intestinal stromal cell lines: response to cytokines and interactions with epithelial cells. Exp Cell Res. 1999 May 1;248(2):391–406. doi: 10.1006/excr.1999.4414. [DOI] [PubMed] [Google Scholar]
- Göke M., Kanai M., Podolsky D. K. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am J Physiol. 1998 May;274(5 Pt 1):G809–G818. doi: 10.1152/ajpgi.1998.274.5.G809. [DOI] [PubMed] [Google Scholar]
- Halttunen T., Marttinen A., Rantala I., Kainulainen H., Mäki M. Fibroblasts and transforming growth factor beta induce organization and differentiation of T84 human epithelial cells. Gastroenterology. 1996 Nov;111(5):1252–1262. doi: 10.1053/gast.1996.v111.pm8898639. [DOI] [PubMed] [Google Scholar]
- Hormi K., Onolfo J. P., Gres L., Lebraud V., Lehy T. Developmental expression of transforming growth factor-alpha in the upper digestive tract and pancreas of the rat. Regul Pept. 1995 Jan 5;55(1):67–77. doi: 10.1016/0167-0115(94)00093-d. [DOI] [PubMed] [Google Scholar]
- Jones B. A., Gores G. J. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997 Dec;273(6 Pt 1):G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- Kaouass M., Deloyer P., Gouders I., Peulen O., Dandrifosse G. Role of interleukin-1 beta, interleukin-6, and TNF-alpha in intestinal maturation induced by dietary spermine in rats. Endocrine. 1997 Apr;6(2):187–194. doi: 10.1007/BF02738963. [DOI] [PubMed] [Google Scholar]
- Keshav S., Lawson L., Chung L. P., Stein M., Perry V. H., Gordon S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J Exp Med. 1990 Jan 1;171(1):327–332. doi: 10.1084/jem.171.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko T. C., Beauchamp R. D., Townsend C. M., Jr, Thompson E. A., Thompson J. C. Transforming growth factor-beta inhibits rat intestinal cell growth by regulating cell cycle specific gene expression. Am J Surg. 1994 Jan;167(1):14–20. doi: 10.1016/0002-9610(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Kurokowa M., Lynch K., Podolsky D. K. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- Laurent-Huck F. M., Egles C., Kienlen P., Stoeckel M. E., Felix J. M. Expression of the c-ets1 gene in the hypothalamus and pituitary during rat development. Brain Res Dev Brain Res. 1996 Nov 22;97(1):107–117. doi: 10.1016/s0165-3806(96)00134-4. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Ciacci C., Podolsky D. K. Peptide growth factors: role in epithelial-lamina propria cell interactions. Ann N Y Acad Sci. 1992;664:148–156. doi: 10.1111/j.1749-6632.1992.tb39757.x. [DOI] [PubMed] [Google Scholar]
- McGee D. W., Vitkus S. J., Lee P. The effect of cytokine stimulation on IL-1 receptor mRNA expression by intestinal epithelial cells. Cell Immunol. 1996 Mar 15;168(2):276–280. doi: 10.1006/cimm.1996.0076. [DOI] [PubMed] [Google Scholar]
- Mengheri E., Ciapponi L., Vignolini F., Nobili F. Cytokine gene expression in intestine of rat during the postnatal developmental period: increased IL-1 expression at weaning. Life Sci. 1996;59(15):1227–1236. doi: 10.1016/0024-3205(96)00446-8. [DOI] [PubMed] [Google Scholar]
- Michalsky M. P., Deitch E. A., Ding J., Lu Q., Huang Q. Interleukin-6 and tumor necrosis factor production in an enterocyte cell model (Caco-2) during exposure to Escherichia coli. Shock. 1997 Feb;7(2):139–146. doi: 10.1097/00024382-199702000-00010. [DOI] [PubMed] [Google Scholar]
- Miettinen P. J., Berger J. E., Meneses J., Phung Y., Pedersen R. A., Werb Z., Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995 Jul 27;376(6538):337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Molmenti E. P., Ziambaras T., Perlmutter D. H. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993 Jul 5;268(19):14116–14124. [PubMed] [Google Scholar]
- Neumann B., Luz A., Pfeffer K., Holzmann B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J Exp Med. 1996 Jul 1;184(1):259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttila I. A., van Spriel A. B., Zhang M. F., Xian C. J., Steeb C. B., Cummins A. G., Zola H., Read L. C. Transforming growth factor-beta levels in maternal milk and expression in postnatal rat duodenum and ileum. Pediatr Res. 1998 Oct;44(4):524–531. doi: 10.1203/00006450-199810000-00010. [DOI] [PubMed] [Google Scholar]
- Plateroti M., Freund J. N., Leberquier C., Kedinger M. Mesenchyme-mediated effects of retinoic acid during rat intestinal development. J Cell Sci. 1997 May;110(Pt 10):1227–1238. doi: 10.1242/jcs.110.10.1227. [DOI] [PubMed] [Google Scholar]
- Plateroti M., Rubin D. C., Duluc I., Singh R., Foltzer-Jourdainne C., Freund J. N., Kedinger M. Subepithelial fibroblast cell lines from different levels of gut axis display regional characteristics. Am J Physiol. 1998 May;274(5 Pt 1):G945–G954. doi: 10.1152/ajpgi.1998.274.5.G945. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol. 1993 Feb;264(2 Pt 1):G179–G186. doi: 10.1152/ajpgi.1993.264.2.G179. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Tian J. Q., Göke M., Podolsky D. K. Glucocorticoids have pleiotropic effects on small intestinal crypt cells. Am J Physiol. 1999 Nov;277(5 Pt 1):G1027–G1040. doi: 10.1152/ajpgi.1999.277.5.G1027. [DOI] [PubMed] [Google Scholar]
- Radema S. A., van Deventer S. J., Cerami A. Interleukin 1 beta is expressed predominantly by enterocytes in experimental colitis. Gastroenterology. 1991 May;100(5 Pt 1):1180–1186. [PubMed] [Google Scholar]
- Roberts D. J., Johnson R. L., Burke A. C., Nelson C. E., Morgan B. A., Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995 Oct;121(10):3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Schaudies R. P., Grimes J., Davis D., Rao R. K., Koldovský O. EGF content in the gastrointestinal tract of rats: effect of age and fasting/feeding. Am J Physiol. 1989 May;256(5 Pt 1):G856–G861. doi: 10.1152/ajpgi.1989.256.5.G856. [DOI] [PubMed] [Google Scholar]
- Simo P., Simon-Assmann P., Arnold C., Kedinger M. Mesenchyme-mediated effect of dexamethasone on laminin in cocultures of embryonic gut epithelial cells and mesenchyme-derived cells. J Cell Sci. 1992 Jan;101(Pt 1):161–171. doi: 10.1242/jcs.101.1.161. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Smith T. J., Blieden T. M., Phipps R. P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997 Aug;151(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- Thompson F. M., Mayrhofer G., Cummins A. G. Dependence of epithelial growth of the small intestine on T-cell activation during weaning in the rat. Gastroenterology. 1996 Jul;111(1):37–44. doi: 10.1053/gast.1996.v111.pm8698223. [DOI] [PubMed] [Google Scholar]
- Youngman K. R., Simon P. L., West G. A., Cominelli F., Rachmilewitz D., Klein J. S., Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993 Mar;104(3):749–758. doi: 10.1016/0016-5085(93)91010-f. [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang L., Duff G. W. A negative regulatory region containing a glucocorticosteroid response element (nGRE) in the human interleukin-1beta gene. DNA Cell Biol. 1997 Feb;16(2):145–152. doi: 10.1089/dna.1997.16.145. [DOI] [PubMed] [Google Scholar]
- Ziambaras T., Rubin D. C., Perlmutter D. H. Regulation of sucrase-isomaltase gene expression in human intestinal epithelial cells by inflammatory cytokines. J Biol Chem. 1996 Jan 12;271(2):1237–1242. doi: 10.1074/jbc.271.2.1237. [DOI] [PubMed] [Google Scholar]