Abstract
BACKGROUND—The vasoactive peptide endothelin 1 (ET-1) acts via two receptors, endothelin receptors A (ETA) and B (ETB). ET-1 is overexpressed by human cancers in vivo and in vitro and may be mitogenic for cancer cells. METHOD—To elucidate if ET-1 is a growth regulator the following were investigated in human colorectal cancer cell lines (LIM1215 and HT29): ET-1 production by ELISA; ET receptor expression using radioligand autoradiographic techniques; and responsiveness to ET-1, and to ETA and ETB antagonism by growth measurements. RESULTS—ET-1 was produced by LIM1215 and HT29 cells (21.3 and 41.7 fmol/ml/106 cells (24 hours); 22.6 and 71.7 fmol/ml/106 cells (48 hours), respectively). ETA and ETB receptors were expressed by both cell lines. Addition of ET-1 resulted in a dose dependent increase in cell numbers which was significant at 10−8-10−9 M for LIM1215, with the greatest increase at 10−8 M (32.7% and 28.4% increase above controls at 48 hours and 72 hours; p<0.05) and at 10−8-10−9 M for HT29, with the greatest increase at 10−9 M (13.4% and 15.7% increase above controls at 48 hours and 72 hours; p<0.05). ETA antagonists BQ123 and BQ610, but not the ETB antagonist BQ788, inhibited ET-1 induced proliferation of both LIM1215 and HT29 (p<0.05). CONCLUSION—ET-1 can stimulate the proliferation of colorectal cancer cell lines via the ETA, but not the ETB, receptor. Keywords: endothelin-1; endothelin receptor; colorectal cancer; colorectal cancer cell lines
Full Text
The Full Text of this article is available as a PDF (144.4 KB).
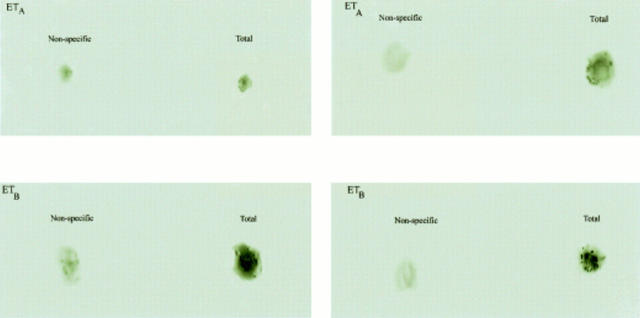
Figure 1 .
Binding for endothelin receptors (ETA and ETB) on LIM1215 (left) and HT29 (right) cell cytospins was demonstrated by autoradiography. Slides were incubated with ETA antagonist (125I PD151242) or ETB agonist (125I BQ3020) for total binding. Non-specific binding was determined by incubation with excess unlabelled ligand. Total binding was clearly in excess of non-specific binding, suggesting the presence of both receptors in these cell types.
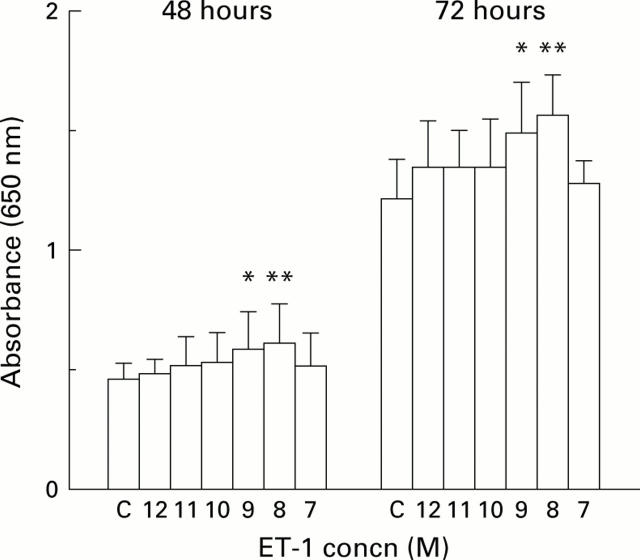
Figure 2 .
Effect of addition of endothelin 1 (ET-1) on LIM1215 cell growth. Cells were grown for 48 or 72 hours in the presence of increasing concentrations of ET-1 (10−12 M to 10−7 M; shown on the x axis as 12 to 7). Cell growth was measured using the methylene blue assay and read as absorbance (equivalent to cell numbers) at 650 nm. Results are shown as mean (SD). Statistically significant growth is shown as *p<0.05 or **p<0.01 (Student's paired t test). C, control.
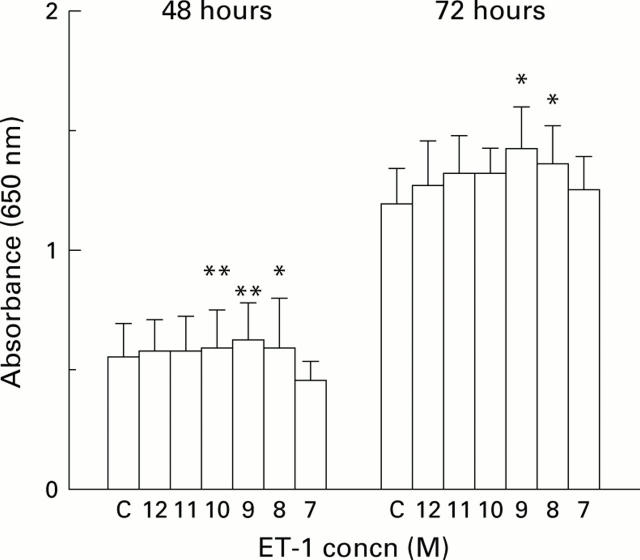
Figure 3 .
Effect of addition of endothelin 1 (ET-1) on HT29 cell growth. Cells were grown for 48 or 72 hours in the presence of increasing concentrations of ET-1 (10−12 M to 10−7 M, shown on the x axis as 12 to 7). Cell growth was measured using the methylene blue assay and read as absorbance (equivalent to cell numbers) at 650 nm. Results are shown as mean (SD). Statistically significant cell growth is shown as *p<0.05 or **p<0.01 (Student's paired t test). C, control.
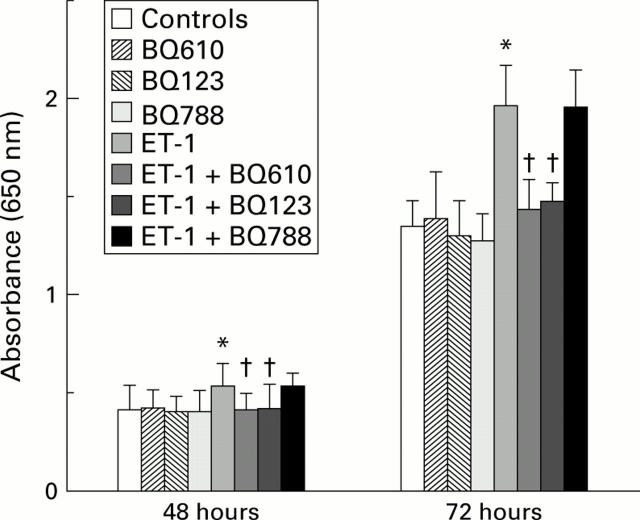
Figure 4 .
Effect of endothelin 1 (ET-1) and/or endothelin receptor antagonists on LIM1215 cell growth. Cells were grown for 48 or 72 hours in the presence of 10−8 M ET-1 and/or ETA receptor antagonist BQ123 or BQ610, or ETB receptor antagonist BQ788, all at 100 nM. Cell growth was measured using the methylene blue assay and read as absorbance (equivalent to cell numbers) at 650 nm. Results are shown as mean (SD). Cell numbers were significantly higher in the ET-1 group compared with control cells (*p<0.05, Student's t test); cell numbers from groups treated with both ET-1 and ETA antagonists were similar to control levels, and were significantly lower than in the ET-1 group (†p<0.05, Student's t test).
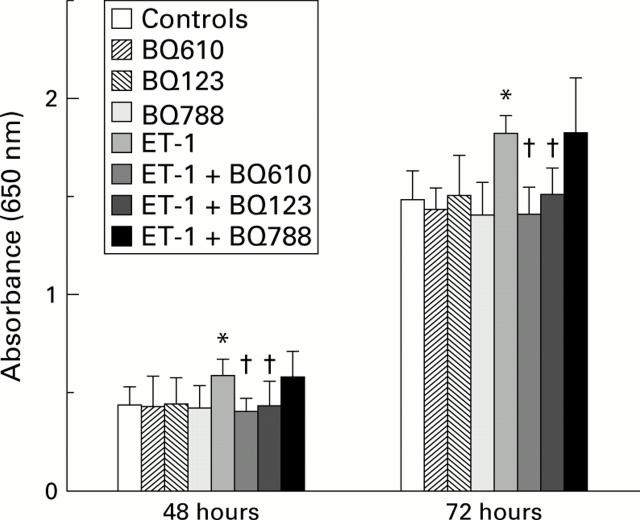
Figure 5 .
Effect of endothelin 1 (ET-1) and/or endothelin receptor antagonists on HT29 cell growth. Cells were grown for 48 or 72 hours in the presence of 10−9 M ET-1 and/or ETA receptor antagonist BQ123 or BQ610, or ETB receptor antagonist BQ788, all at 100 nM. Cell growth was measured using the methylene blue assay and read as absorbance (equivalent to cell numbers) at 650 nm. Results are shown as mean (SD). Cell numbers were significantly higher in the ET-1 group compared with control cells (*p<0.05, Student's t-test); cell numbers from groups treated with both ET-1 and ETA antagonists were similar to control levels, and were significantly lower than in the ET-1 group (†p<0.05, Student's t test).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Bagnato A., Salani D., Di Castro V., Wu-Wong J. R., Tecce R., Nicotra M. R., Venuti A., Natali P. G. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999 Feb 1;59(3):720–727. [PubMed] [Google Scholar]
- Bagnato A., Tecce R., Di Castro V., Catt K. J. Activation of mitogenic signaling by endothelin 1 in ovarian carcinoma cells. Cancer Res. 1997 Apr 1;57(7):1306–1311. [PubMed] [Google Scholar]
- Baley P. A., Resink T. J., Eppenberger U., Hahn A. W. Endothelin messenger RNA and receptors are differentially expressed in cultured human breast epithelial and stromal cells. J Clin Invest. 1990 Apr;85(4):1320–1323. doi: 10.1172/JCI114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood M. R., Timm M., Kaski J. C. Regional variations in ETA/ETB binding sites in human coronary vasculature. J Cardiovasc Pharmacol. 1995;26 (Suppl 3):S351–S354. [PubMed] [Google Scholar]
- Dashwood M. R., Timm M., Muddle J. R., Ong A. C., Tippins J. R., Parker R., McManus D., Murday A. J., Madden B. P., Kaski J. C. Regional variations in endothelin-1 and its receptor subtypes in human coronary vasculature: pathophysiological implications in coronary disease. Endothelium. 1998;6(1):61–70. doi: 10.3109/10623329809053405. [DOI] [PubMed] [Google Scholar]
- Fujitani Y., Ninomiya H., Okada T., Urade Y., Masaki T. Suppression of endothelin-1-induced mitogenic responses of human aortic smooth muscle cells by interleukin-1 beta. J Clin Invest. 1995 Jun;95(6):2474–2482. doi: 10.1172/JCI117948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaid A., Hamid Q. A., Springall D. R., Yanagisawa M., Shinmi O., Sawamura T., Masaki T., Kimura S., Corrin B., Polak J. M. Detection of endothelin immunoreactivity and mRNA in pulmonary tumours. J Pathol. 1990 Sep;162(1):15–22. doi: 10.1002/path.1711620105. [DOI] [PubMed] [Google Scholar]
- Hackel P. O., Zwick E., Prenzel N., Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999 Apr;11(2):184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- Inagaki H., Bishop A. E., Eimoto T., Polak J. M. Autoradiographic localization of endothelin-1 binding sites in human colonic cancer tissue. J Pathol. 1992 Nov;168(3):263–267. doi: 10.1002/path.1711680304. [DOI] [PubMed] [Google Scholar]
- Inagaki H., Bishop A. E., Escrig C., Wharton J., Allen-Mersh T. G., Polak J. M. Localization of endothelinlike immunoreactivity and endothelin binding sites in human colon. Gastroenterology. 1991 Jul;101(1):47–54. doi: 10.1016/0016-5085(91)90458-w. [DOI] [PubMed] [Google Scholar]
- Inagaki H., Bishop A. E., Yura J., Polak J. M. Localization of endothelin-1 and its binding sites to the nervous system of the human colon. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S455–S457. doi: 10.1097/00005344-199100177-00131. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M., Fujita M., Nagai K., Kako M., Furue H., Haku E., Osamura Y., Yamaji T. Production and secretion of endothelin by hepatocellular carcinoma. J Clin Endocrinol Metab. 1993 Feb;76(2):378–383. doi: 10.1210/jcem.76.2.7679399. [DOI] [PubMed] [Google Scholar]
- Jansen B., Uckun F. M., Jaszcz W. B., Kersey J. H. Establishment of a human t(4;11) leukemia in severe combined immunodeficient mice and successful treatment using anti-CD19 (B43)-pokeweed antiviral protein immunotoxin. Cancer Res. 1992 Jan 15;52(2):406–412. [PubMed] [Google Scholar]
- Kojima K., Nihei Z. Expression of endothelin-1 immunoreactivity in breast cancer. Surg Oncol. 1995;4(6):309–315. doi: 10.1016/s0960-7404(10)80043-x. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurihara H., Sugiyama T., Yoshizumi M., Takaku F., Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988 Oct 10;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Kusuhara M., Yamaguchi K., Nagasaki K., Hayashi C., Suzaki A., Hori S., Handa S., Nakamura Y., Abe K. Production of endothelin in human cancer cell lines. Cancer Res. 1990 Jun 1;50(11):3257–3261. [PubMed] [Google Scholar]
- Kusuhara M., Yamaguchi K., Ohnishi A., Abe K., Kimura S., Oono H., Hori S., Nakamura Y. Endothelin potentiates growth factor-stimulated DNA synthesis in Swiss 3T3 cells. Jpn J Cancer Res. 1989 Apr;80(4):302–305. doi: 10.1111/j.1349-7006.1989.tb02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L. M., Daaka Y., Lefkowitz R. J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999 Apr;11(2):177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- Mathieu M. N., Chevillard C. Endothelin-1 and ETA receptor subtype are expressed in the gastric HGT-1 cell line. J Cardiovasc Pharmacol. 1995;26 (Suppl 3):S508–S509. [PubMed] [Google Scholar]
- Moraitis S., Langdon S. P., Miller W. R. Endothelin expression and responsiveness in human ovarian carcinoma cell lines. Eur J Cancer. 1997 Apr;33(4):661–668. doi: 10.1016/s0959-8049(97)00012-9. [DOI] [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Yamamoto S., Kato R. Endothelin-mediated stimulation of DNA synthesis in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Feb 15;158(3):880–883. doi: 10.1016/0006-291x(89)92804-0. [DOI] [PubMed] [Google Scholar]
- Nelson J. B., Chan-Tack K., Hedican S. P., Magnuson S. R., Opgenorth T. J., Bova G. S., Simons J. W. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996 Feb 15;56(4):663–668. [PubMed] [Google Scholar]
- Oikawa T., Kushuhara M., Ishikawa S., Hitomi J., Kono A., Iwanaga T., Yamaguchi K. Production of endothelin-1 and thrombomodulin by human pancreatic cancer cells. Br J Cancer. 1994 Jun;69(6):1059–1064. doi: 10.1038/bjc.1994.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver M. H., Harrison N. K., Bishop J. E., Cole P. J., Laurent G. J. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci. 1989 Mar;92(Pt 3):513–518. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Saville M. K., Graham A., Malarkey K., Paterson A., Gould G. W., Plevin R. Regulation of endothelin-1- and lysophosphatidic acid-stimulated tyrosine phosphorylation of focal adhesion kinase (pp125fak) in Rat-1 fibroblasts. Biochem J. 1994 Jul 15;301(Pt 2):407–414. doi: 10.1042/bj3010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A., Loizidou M., Aliev G., Fredericks S., Holt D., Boulos P. B., Burnstock G., Taylor I. Raised endothelin 1 levels in patients with colorectal liver metastases. Br J Surg. 1998 Apr;85(4):502–506. doi: 10.1046/j.1365-2168.1998.00660.x. [DOI] [PubMed] [Google Scholar]
- Shichiri M., Hirata Y., Nakajima T., Ando K., Imai T., Yanagisawa M., Masaki T., Marumo F. Endothelin-1 is an autocrine/paracrine growth factor for human cancer cell lines. J Clin Invest. 1991 May;87(5):1867–1871. doi: 10.1172/JCI115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden P. D., Ittmann M., Monaco M. E., Lepor H. Endothelin-1 production and agonist activities in cultured prostate-derived cells: implications for regulation of endothelin bioactivity and bioavailability in prostatic hyperplasia. Prostate. 1998 Mar 1;34(4):241–250. doi: 10.1002/(sici)1097-0045(19980301)34:4<241::aid-pros1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]