Abstract
AIM—This study describes the long term follow up of haemophilic patients infected with hepatitis C virus (HCV) between 1961 and 1985. METHODS—Clinical and treatment records from 310 patients with inherited coagulation disorders treated with blood product before 1985 were reviewed. Standard survival analysis methods were used to model progression to liver failure and death. RESULTS—A total of 298/305 (98%) patients tested were anti-HCV positive. Twenty seven (9%) individuals consistently HCV polymerise chain reaction negative were considered to have cleared the virus. By 1 September 1999, 223/310 (72%) were alive, 26 (8%) had died a liver related death, and 61 (20%) had died from other, predominantly human immunodeficiency virus (HIV) related, causes. Kaplan-Meier progression rates to death from any cause and liver related deaths 25 years after exposure to HCV were 47% (95% confidence intervals (CI) 34-60) and 19% (95% CI 10-27), respectively. After 13.3 years from 1985, by which time all patients had seroconverted to HIV, progression rates to death from any cause and liver related deaths were, respectively, 8% (95% CI 4-13) and 3% (95% CI 0.4-6) for those HIV negative, and 57% (95% CI 48-66) and 21% (95% CI 13-31) for those HIV positive (p=0.0001). Using Cox proportional hazard models, the adjusted relative hazard of death for individuals coinfected with HIV compared with those infected with HCV alone was 19.47 (95% CI 9.22-41.10), 0.99 (95% CI 0.39-2.53), 3.47 (95% CI 1.40-8.63), and 9.74 (95% CI 3.91-24.26) for the age groups at infection 10-19 years, 20-29 years, and >30 years, respectively, compared with the age group <10 years. The adjusted relative hazard for genotype 1 compared with other genotypes was 2.7 (95% CI 1.36-5.15) . CONCLUSIONS—While 25 year follow up of 310 haemophilic patients has shown the potentially lethal combination of HIV and HCV coinfection, HCV singly infected individuals show slow progression of liver disease. Keywords: hepatitis C virus; human immunodeficiency virus; haemophilia
Full Text
The Full Text of this article is available as a PDF (134.4 KB).
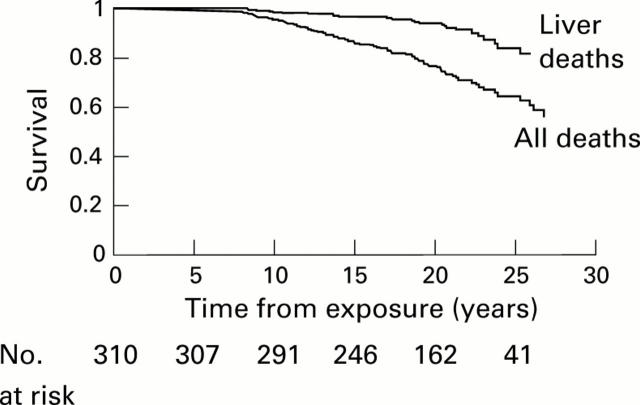
Figure 1 .
Kaplan-Meier progression rates to death from any cause and deaths related to liver disease, yearly after exposure to hepatitis C virus.
Figure 2 .
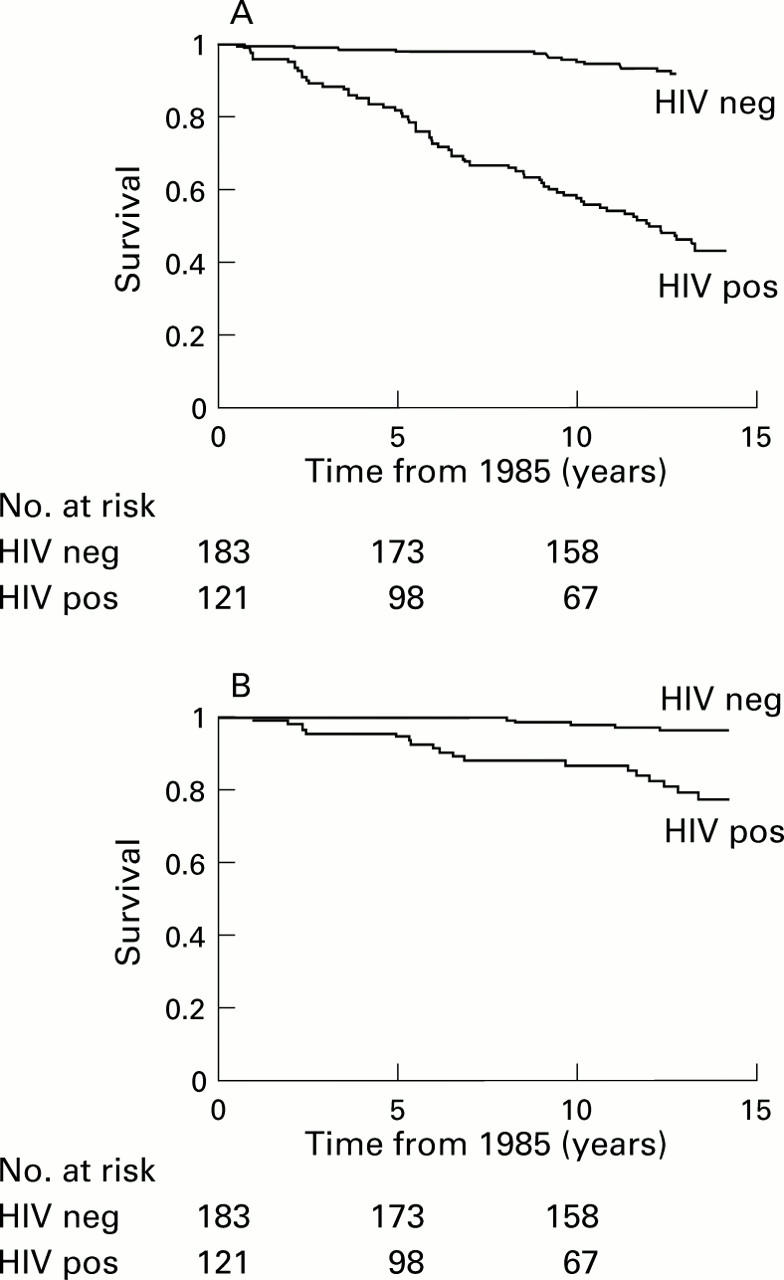
(A) Kaplan-Meier progression rate to death from any cause from 1985 in human immunodeficiency virus (HIV) positive and negative patients. (B) Kaplan-Meier progression rate to liver related death from 1985 in HIV positive and negative patients.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamber M., Murray A., Arborgh B. A., Scheuer P. J., Kernoff P. B., Thomas H. C., Sherlock S. Short incubation non-A, non-B hepatitis transmitted by factor VIII concentrates in patients with congenital coagulation disorders. Gut. 1981 Oct;22(10):854–859. doi: 10.1136/gut.22.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E., Dormandy K. M., Churchill W. G., Coward A. R., Smith M., Cleghorn T. E. Cryoprecipitate and the plastic blood-bag system: provision of adequate replacement therapy for routine treatment of haemophilia. Br Med J. 1967 Apr 8;2(5544):88–91. doi: 10.1136/bmj.2.5544.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton-Maggs P. H., Wensley R. T., Kernoff P. B., Kasper C. K., Winkelman L., Lane R. S., Smith J. K. Production and therapeutic use of a factor XI concentrate from plasma. Thromb Haemost. 1992 Mar 2;67(3):314–319. [PubMed] [Google Scholar]
- Darby S. C., Ewart D. W., Giangrande P. L., Spooner R. J., Rizza C. R., Dusheiko G. M., Lee C. A., Ludlam C. A., Preston F. E. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. UK Haemophilia Centre Directors' Organisation. Lancet. 1997 Nov 15;350(9089):1425–1431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- Dittmann S., Roggendorf M., Dürkop J., Wiese M., Lorbeer B., Deinhardt F. Long-term persistence of hepatitis C virus antibodies in a single source outbreak. J Hepatol. 1991 Nov;13(3):323–327. doi: 10.1016/0168-8278(91)90076-n. [DOI] [PubMed] [Google Scholar]
- Dusheiko G., Main J., Thomas H., Reichard O., Lee C., Dhillon A., Rassam S., Fryden A., Reesink H., Bassendine M. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996 Nov;25(5):591–598. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- Dusheiko G., Schmilovitz-Weiss H., Brown D., McOmish F., Yap P. L., Sherlock S., McIntyre N., Simmonds P. Hepatitis C virus genotypes: an investigation of type-specific differences in geographic origin and disease. Hepatology. 1994 Jan;19(1):13–18. [PubMed] [Google Scholar]
- Eyster M. E., Diamondstone L. S., Lien J. M., Ehmann W. C., Quan S., Goedert J. J. Natural history of hepatitis C virus infection in multitransfused hemophiliacs: effect of coinfection with human immunodeficiency virus. The Multicenter Hemophilia Cohort Study. J Acquir Immune Defic Syndr. 1993 Jun;6(6):602–610. [PubMed] [Google Scholar]
- Eyster M. E., Fried M. W., Di Bisceglie A. M., Goedert J. J. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994 Aug 15;84(4):1020–1023. [PubMed] [Google Scholar]
- Eyster M. E., Sherman K. E., Goedert J. J., Katsoulidou A., Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis. 1999 May;179(5):1062–1069. doi: 10.1086/314708. [DOI] [PubMed] [Google Scholar]
- Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999 Apr 22;340(16):1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- Kernoff P. B., Lee C. A., Karayiannis P., Thomas H. C. High risk of non-A non-B hepatitis after a first exposure to volunteer or commercial clotting factor concentrates: effects of prophylactic immune serum globulin. Br J Haematol. 1985 Jul;60(3):469–479. doi: 10.1111/j.1365-2141.1985.tb07444.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Tanaka E., Sodeyama T., Urushihara A., Matsumoto A., Kiyosawa K. The natural course of chronic hepatitis C: a comparison between patients with genotypes 1 and 2 hepatitis C viruses. Hepatology. 1996 Apr;23(4):695–699. doi: 10.1053/jhep.1996.v23.pm0008666319. [DOI] [PubMed] [Google Scholar]
- Lee C. A. Investigation of chronic hepatitis C infection in individuals with haemophilia. Br J Haematol. 1997 Feb;96(2):425–426. doi: 10.1046/j.1365-2141.1997.d01-2007.x. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Kernoff P. B., Karayiannis P., Thomas H. C. Acute fulminant non-A, non-B hepatitis leading to chronic active hepatitis after treatment with cryoprecipitate. Gut. 1985 Jun;26(6):639–641. doi: 10.1136/gut.26.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Kernoff P. B., Karayiannis P., Thomas H. C. Interferon therapy for chronic non-A non-B and chronic delta liver disease in haemophilia. Br J Haematol. 1989 Jun;72(2):235–238. doi: 10.1111/j.1365-2141.1989.tb07688.x. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Phillips A., Elford J., Miller E. J., Bofill M., Griffiths P. D., Kernoff P. B. The natural history of human immunodeficiency virus infection in a haemophilic cohort. Br J Haematol. 1989 Oct;73(2):228–234. doi: 10.1111/j.1365-2141.1989.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Martin P., Di Bisceglie A. M., Kassianides C., Lisker-Melman M., Hoofnagle J. H. Rapidly progressive non-A, non-B hepatitis in patients with human immunodeficiency virus infection. Gastroenterology. 1989 Dec;97(6):1559–1561. doi: 10.1016/0016-5085(89)90405-8. [DOI] [PubMed] [Google Scholar]
- Mauser-Bunschoten E. P., Bresters D., van Drimmelen A. A., Roosendaal G., Cuypers H. T., Reesink H. W., van der Poel C. L., van den Berg H. M., Lelie P. N. Hepatitis C infection and viremia in Dutch hemophilia patients. J Med Virol. 1995 Mar;45(3):241–246. doi: 10.1002/jmv.1890450302. [DOI] [PubMed] [Google Scholar]
- McHutchison J. G., Gordon S. C., Schiff E. R., Shiffman M. L., Lee W. M., Rustgi V. K., Goodman Z. D., Ling M. H., Cort S., Albrecht J. K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998 Nov 19;339(21):1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- Mendenhall C. L., Seeff L., Diehl A. M., Ghosn S. J., French S. W., Gartside P. S., Rouster S. D., Buskell-Bales Z., Grossman C. J., Roselle G. A. Antibodies to hepatitis B virus and hepatitis C virus in alcoholic hepatitis and cirrhosis: their prevalence and clinical relevance. The VA Cooperative Study Group (No. 119) Hepatology. 1991 Oct;14(4 Pt 1):581–589. doi: 10.1016/0270-9139(91)90042-t. [DOI] [PubMed] [Google Scholar]
- Merkel C., Sacerdoti D., Bolognesi M., Enzo E., Marin R., Bombonato G., Angeli P., Gatta A. Hemodynamic evaluation of the addition of isosorbide-5-mononitrate to nadolol in cirrhotic patients with insufficient response to the beta-blocker alone. Hepatology. 1997 Jul;26(1):34–39. doi: 10.1053/jhep.1997.v26.pm0009214449. [DOI] [PubMed] [Google Scholar]
- Nguyen T. T., Sedghi-Vaziri A., Wilkes L. B., Mondala T., Pockros P. J., Lindsay K. L., McHutchison J. G. Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection. J Viral Hepat. 1996 Mar;3(2):75–78. doi: 10.1111/j.1365-2893.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S., Kuroki T., Yabusako T., Seki S., Kobayashi K., Monna T., Otani S., Sakurai M., Shikata T., Yamamoto S. Detection of hepatitis C virus antibodies and hepatitis C virus RNA in patients with alcoholic liver disease. Hepatology. 1991 Dec;14(6):985–989. [PubMed] [Google Scholar]
- Nitu I. C., Lee C. A., Dhillon A. P., Mistry P. K. Management of liver failure in a haemophilic patient co-infected with human immunodeficiency and hepatitis C viruses. Clin Lab Haematol. 1999 Apr;21(2):139–141. doi: 10.1046/j.1365-2257.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- Poynard T., Marcellin P., Lee S. S., Niederau C., Minuk G. S., Ideo G., Bain V., Heathcote J., Zeuzem S., Trepo C. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998 Oct 31;352(9138):1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- Sabin C. A., Telfer P., Phillips A. N., Bhagani S., Lee C. A. The association between hepatitis C virus genotype and human immunodeficiency virus disease progression in a cohort of hemophilic men. J Infect Dis. 1997 Jan;175(1):164–168. doi: 10.1093/infdis/175.1.164. [DOI] [PubMed] [Google Scholar]
- Schoeman M. N., Liddle C., Bilous M., Grierson J., Craig P. I., Batey R. G., Farrell G. C. Chronic non-A, non-B hepatitis: lack of correlation between biochemical and morphological activity, and effects of immunosuppressive therapy on disease progression. Aust N Z J Med. 1990 Feb;20(1):56–62. doi: 10.1111/j.1445-5994.1990.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Soto B., Sánchez-Quijano A., Rodrigo L., del Olmo J. A., García-Bengoechea M., Hernández-Quero J., Rey C., Abad M. A., Rodríguez M., Sales Gilabert M. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997 Jan;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- Telfer P. T., Brown D., Devereux H., Lee C. A., DuSheiko G. M. HCV RNA levels and HIV infection: evidence for a viral interaction in haemophilic patients. Br J Haematol. 1994 Oct;88(2):397–399. doi: 10.1111/j.1365-2141.1994.tb05038.x. [DOI] [PubMed] [Google Scholar]
- Telfer P., Sabin C., Devereux H., Scott F., Dusheiko G., Lee C. The progression of HCV-associated liver disease in a cohort of haemophilic patients. Br J Haematol. 1994 Jul;87(3):555–561. doi: 10.1111/j.1365-2141.1994.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Vogt M., Lang T., Frösner G., Klingler C., Sendl A. F., Zeller A., Wiebecke B., Langer B., Meisner H., Hess J. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999 Sep 16;341(12):866–870. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- Yamada G., Tanaka E., Miura T., Kiyosawa K., Yano M., Matsushima T., Tsubouchi H., Ishikawa K., Kohara M., Hino K. Epidemiology of genotypes of hepatitis C virus in Japanese patients with type C chronic liver diseases: a multi-institution analysis. J Gastroenterol Hepatol. 1995 Sep-Oct;10(5):538–545. doi: 10.1111/j.1440-1746.1995.tb01344.x. [DOI] [PubMed] [Google Scholar]