Abstract
BACKGROUND AND AIMS—Inflammatory bowel disease is characterised by oxidative and nitrosative stress, leucocyte infiltration, upregulation of expression of intercellular adhesion molecule 1 (ICAM-1), and upregulation of P-selectin in the colon. The aim of the present study was to examine the effects of calpain inhibitor I in rats subjected to experimental colitis. METHODS—Colitis was induced in rats by intracolonic instillation of dinitrobenzene sulphonic acid (DNBS). RESULTS—Rats experienced haemorrhagic diarrhoea and weight loss. Four days after administration of DNAB, the mucosa of the colon exhibited large areas of necrosis. Neutrophil infiltration (determined by histology as well as by an increase in myeloperoxidase activity in the mucosa) was associated with upregulation of ICAM-1 and P-selectin as well as high tissue levels of malondialdehyde. Immunohistochemistry for nitrotyrosine and poly (ADP-ribose) polymerase (PARP) showed intense staining in the inflamed colon. Staining of sections of colon obtained from DNBS treated rats with an anti-cyclooxygenase 2 antibody showed diffuse staining of the inflamed tissue. Furthermore, expression of inducible nitric oxide synthase was found mainly in macrophages located within the inflamed colon of DNBS treated rats. Calpain inhibitor I (5 mg/kg daily intraperitoneally) significantly reduced the degree of haemorrhagic diarrhoea and weight loss caused by administration of DNBS. Calpain inhibitor I also caused a substantial reduction in (i) degree of colon injury, (ii) rise in myeloperoxidase activity (mucosa), (iii) increase in tissue levels of malondialdehyde, (iv) increase in staining (immunohistochemistry) for nitrotyrosine and PARP, as well as (v) upregulation of ICAM-1 and P-selectin caused by DNBS in the colon. CONCLUSION—Calpain inhibitor I reduces the degree of colitis caused by DNBS. We propose that calpain inhibitor I may be useful in the treatment of inflammatory bowel disease. Keywords: calpain; calpain inhibitor I; cyclooxygenase; nitric oxide; inflammatory bowel disease; rat
Full Text
The Full Text of this article is available as a PDF (368.2 KB).
Figure 1 .
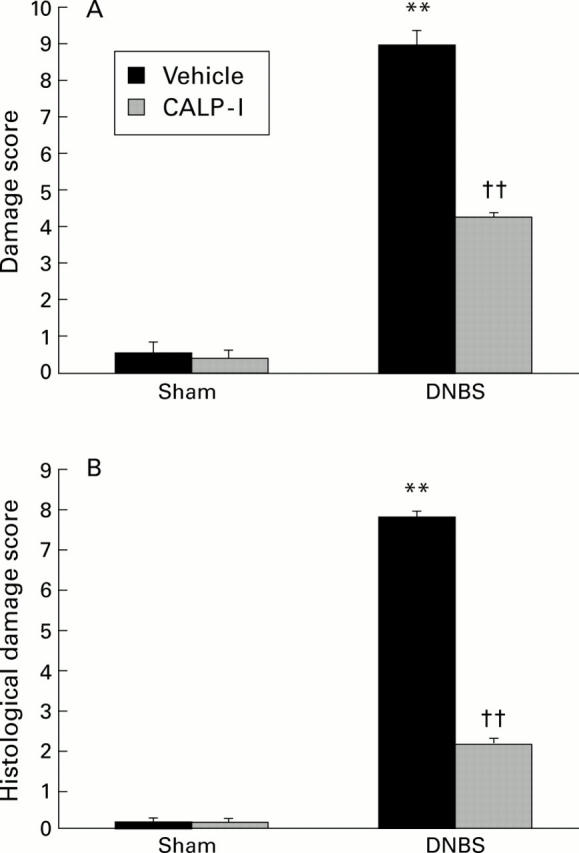
Effects of calpain inhibitor I (CALP-1) on the macroscopic damage score (A) and microscopic histological score (B). Colon damage was scored by two independent observers. Values are mean (SEM) of data obtained from 10 rats in each group. **p<0.01 v sham; ††p<0.01 v dinitrobenzene sulphonic acid (DNBS).
Figure 2 .
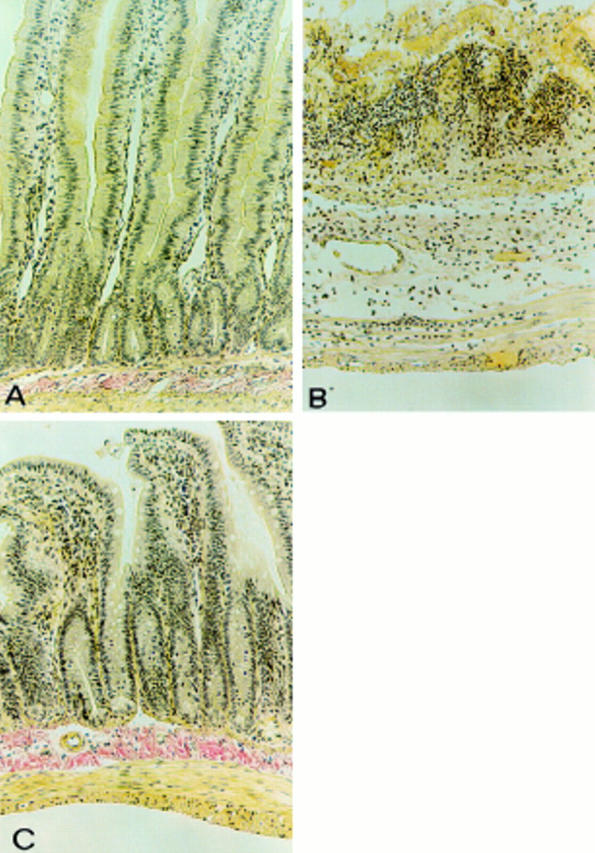
Effects of calpain inhibitor I on colon injury. No histological modification was observed in the mucosa of sham operated rats (A). Mucosal injury was produced after dinitrobenzene sulphonic acid (DNBS) administration, characterised by the absence of epithelium and massive mucosal and submucosal infiltration with inflammatory cells (B). Treatment with calpain inhibitor I (C) corrected the disturbances in morphology associated with DNBS administration. Original magnification ×120. The figure is representative of at least three experiments performed on different experimental days.
Figure 3 .
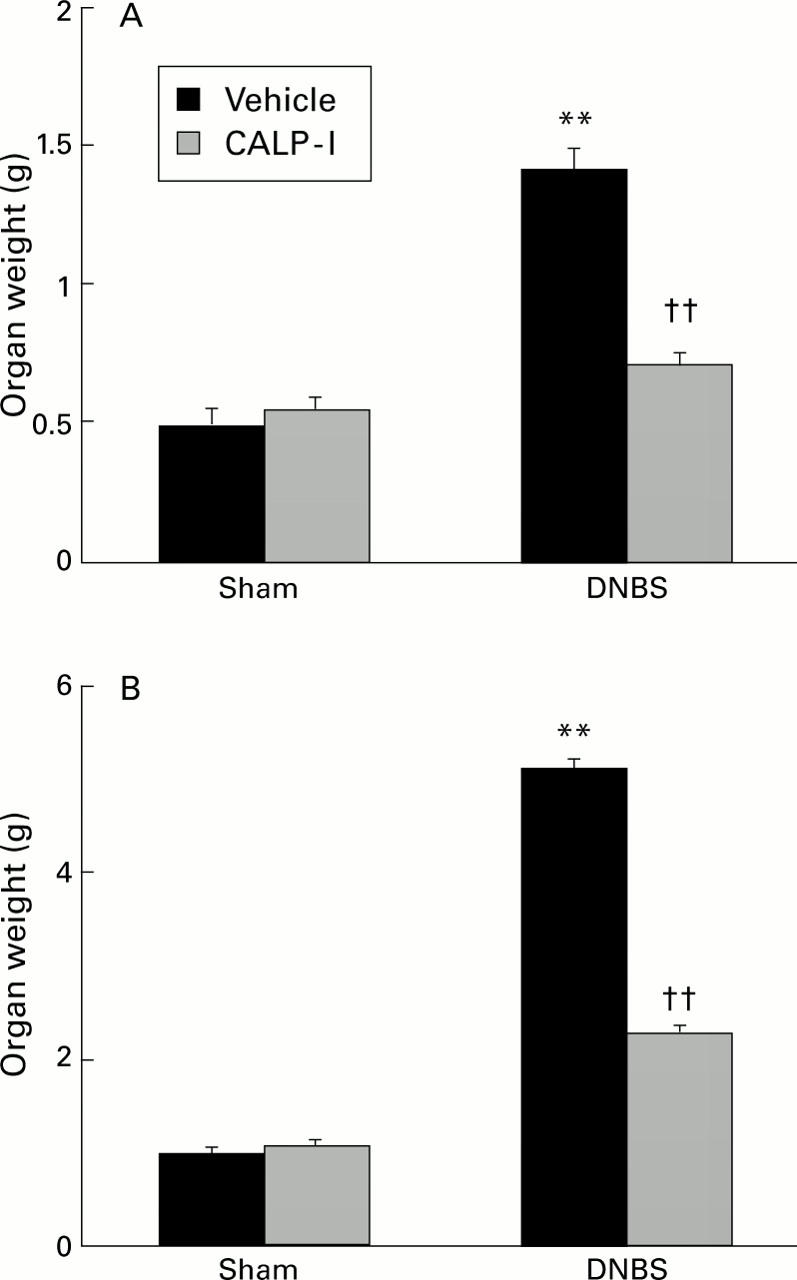
Organ weight. A significant increase in weight in the spleen (A) and colon (B) was consistently seen four days after dinitrobenzene sulphonic acid (DNBS) injection. The weight of the organs was significantly reduced in rats who had been treated with calpain inhibitor I (CALP-1). Values are mean (SEM) of 10 rats in each group. **p<0.01 v sham; ††p<0.01 v DNBS.
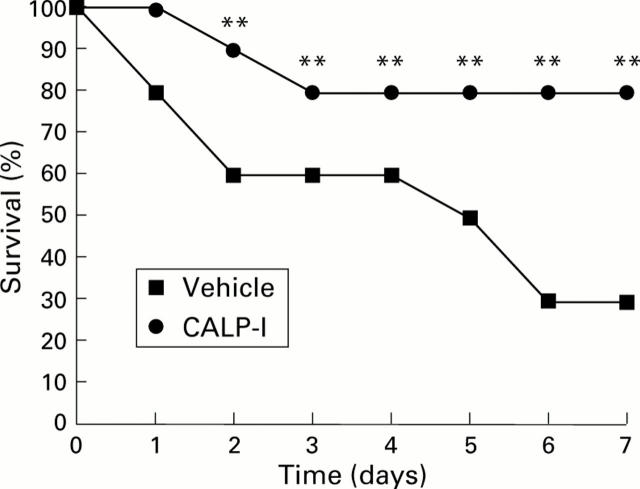
Figure 4 .
Effect of calpain inhibitor I (CALP-1) treatment on dinitrobenzene sulphonic acid (DNBS) induced mortality. Survival is significantly improved in CALP-1 treated rats in comparison with the high mortality rate of the DNBS treated rats. n=10 rats in each group. **p<0.01 v DNBS.
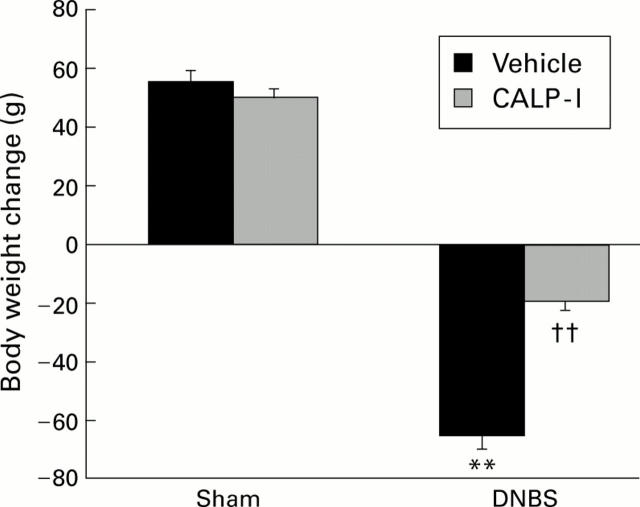
Figure 5 .
Effect of calpain inhibitor I (CALP-1) treatment on changes in body weight four days after dinitrobenzene sulphonic acid (DNBS) intracolonic administration. Body weight was recorded immediately before DNBS administration and at the end of the experimental period. CALP-1 treatment significantly prevented the loss in body weight. Values are mean (SEM) of 10 rats in each group. **p<0.01 v sham; ††p<0.01 v DNBS.
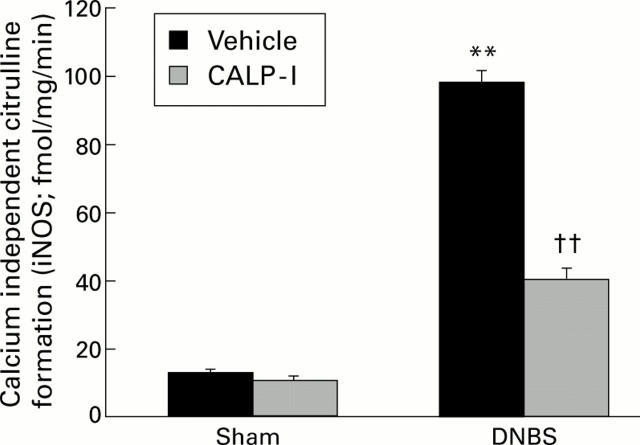
Figure 6 .
Effect of calpain inhibitor I (CALP-1) treatment on inducible nitric oxide synthase (iNOS) activity four days after dinitrobenzene sulphonic acid (DNBS) intracolonic administration. DNBS administration induced a significant increase in iNOS activity. CALP-1 treatment significantly reduced iNOS activity. Values are mean (SEM) of 10 rats in each group. **p<0.01 v sham; ††p<0.01 v DNBS.
Figure 7 .
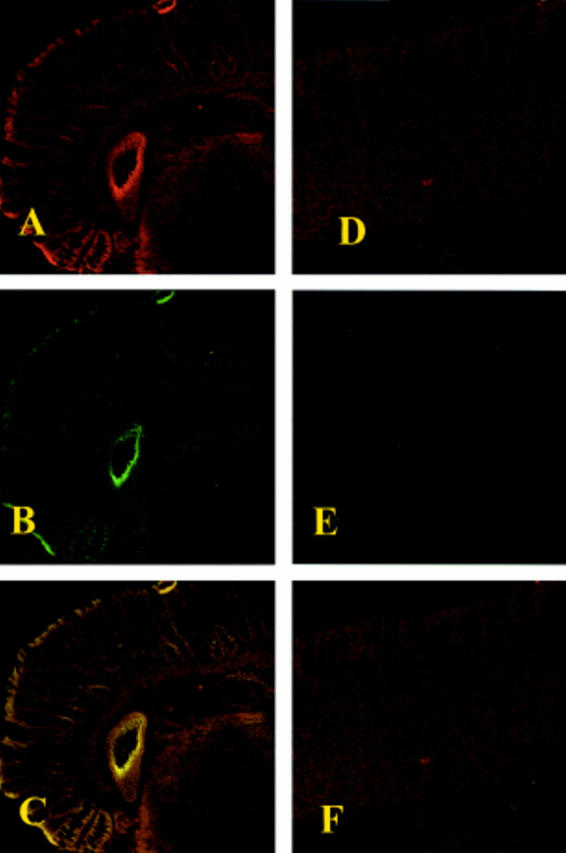
Immunohistochemical localisation for cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) in the colon. Immunohistochemical staining for COX (A) and iNOS (B) showed positive staining localised in the injured area from dinitrobenzene sulphonic acid (DNBS) treated rats. The intensity of the observed staining for COX (D) and iNOS (E) was significantly reduced in the colon from calpain inhibitor I treated rats. (C) and (F) represent the staining combination of (A-B) and (D-E), respectively. Original magnification ×145. The figure is representative of at least three experiments performed on different experimental days.
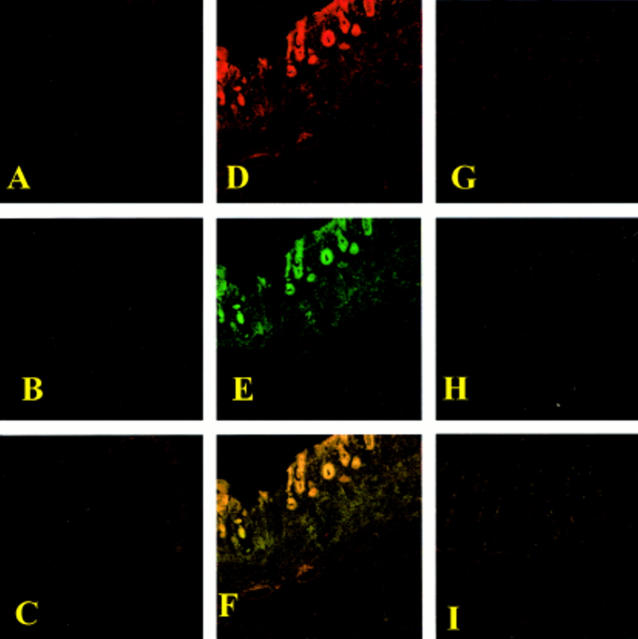
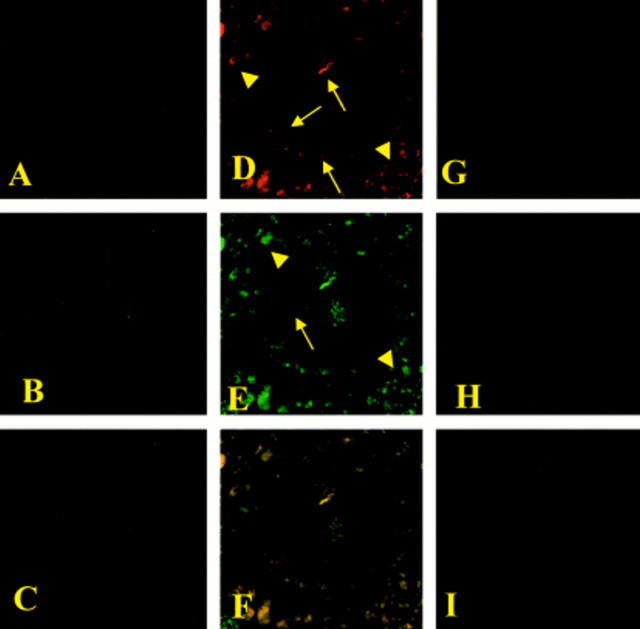
Figure 8 .
Immunohistochemical localisation of nitrotyrosine and poly (ADP-ribose) polymerase (PARP) in the colon. No positive staining for nitrotyrosine (A) or PARP (B) was found in colon sections from sham operated rats. Immunohistochemical staining for nitrotyrosine (D) and PARP (E) showed positive staining localised in the injured area from a dinitrobenzene sulphonic acid (DNBS) treated rat. The intensity of the staining for nitrotyrosine (G) and PARP (H) was significantly reduced in the ileum from calpain inhibitor I treated rats. (C), (F), and (I) represent the staining combination of (A-B), (D-E), and (G-H), respectively. Original magnification ×145. The figure is representative of at least three experiments performed on different experimental days.
Figure 9 .
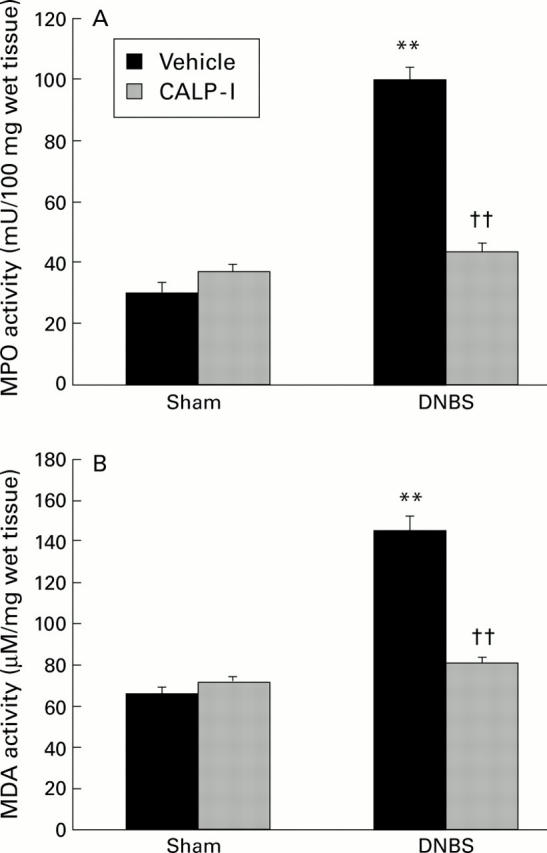
Effect of calpain inhibitor I (CALP-1) on neutrophil infiltration and lipid peroxidation. Myeloperoxidase (MPO) activity (A) and malondialdehyde (MDA) levels (B) in the colon from dinitrobenzene sulphonic acid (DNBS) treated rats. MPO activity and MDA levels were significantly increased in DNBS treated rats in comparison with sham controls. CALP-1 treated rats showed a significant reduction in MPO activity and MDA levels. Values are mean (SEM) of 10 rats in each group. **p<0.01 v sham; ††p<0.01 v DNBS.
Figure 10 .
Immunohistochemical localisation of P-selectin in the colon. Staining of colon sections obtained from sham operated rats with anti-intercellular adhesion molecule 1 (ICAM-1) antibody showed specific staining along vessels, demonstrating that ICAM-1 is constitutively expressed (A). Ileum sections from sham operated rats revealed no positive staining for P-selectin (B). Sections obtained from dinitrobenzene sulphonic acid (DNBS) treated rats showed intense positive staining for ICAM-1 (D) and P-selectin (E) on endothelial cells. The degree of endothelial staining for ICAM-1 (G) and P-selectin (H) was markedly reduced in tissue sections obtained from calpain inhibitor I treated rats. (C), (F), and (I) represent the staining combination of (A-B), (D-E), and (G-H) respectively. Original magnification ×145. The figure is representative of at least three experiments performed on different experimental days.
Figure 11 .
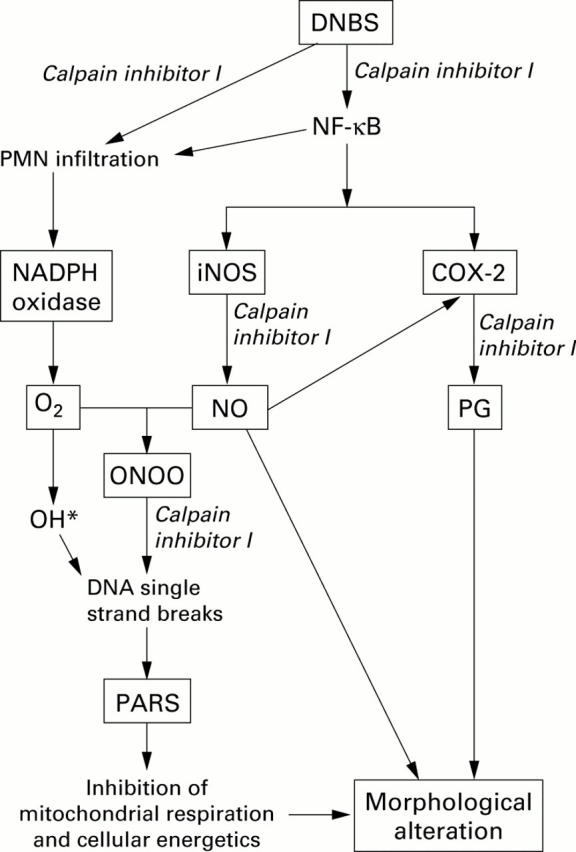
Proposed scheme of some of the delayed inflammatory pathways involving nitric oxide (NO*), hydroxyl radical (OH*), and peroxynitrite (ONOO) in dinitrobenzene sulphonic acid (DNBS) induced colitis, and potential sites of the anti-inflammatory actions of calpain inhibitor I. DNBS triggers expression of inducible nitric oxide (NO) synthase (iNOS), at least in part, via activation of nuclear factor κB (NF-κB). NO in turn combines with superoxide to form ONOO-. Hydroxyl radical (produced from superoxide via the iron catalysed Haber-Weiss reaction) and ONOO- or peroxynitrous acid (ONOOH) induce cellular injury. Part of the injury is related to the development of DNA single strand breakage, with consequent activation of poly (ADP-ribose) polymerase (PARP), leading to cellular dysfunction. Expression of the inducible isoform of cyclooxygenase (COX-2) is also dependent on activation of NF-κB. In addition, NO can directly increase the catalytic activity of COX-2, leading to enhanced production of proinflammatory prostaglandin metabolites. We propose that the anti-inflammatory effects of calpain inhibitor I may include (1) inhibition of activation of NF-κB and prevention of expression of iNOS and COX-2, (2) inhibition of ONOO formation, and (3) prevention of activation of PARP (see discussion for further explanations).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiko S., Grisham M. B. Spontaneous intestinal inflammation and nitric oxide metabolism in HLA-B27 transgenic rats. Gastroenterology. 1995 Jul;109(1):142–150. doi: 10.1016/0016-5085(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Atsma D. E., Bastiaanse E. M., Jerzewski A., Van der Valk L. J., Van der Laarse A. Role of calcium-activated neutral protease (calpain) in cell death in cultured neonatal rat cardiomyocytes during metabolic inhibition. Circ Res. 1995 Jun;76(6):1071–1078. doi: 10.1161/01.res.76.6.1071. [DOI] [PubMed] [Google Scholar]
- Atsma D. E., Bastiaanse E. M., Jerzewski A., Van der Valk L. J., Van der Laarse A. Role of calcium-activated neutral protease (calpain) in cell death in cultured neonatal rat cardiomyocytes during metabolic inhibition. Circ Res. 1995 Jun;76(6):1071–1078. doi: 10.1161/01.res.76.6.1071. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Elliott P. J., Hayward N. J., Dean R. L., Harbeson S., Straub J. A., Li Z., Powers J. C. Calpain as a novel target for treating acute neurodegenerative disorders. Neurol Res. 1995 Aug;17(4):249–258. doi: 10.1080/01616412.1995.11740322. [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Elliott P. J., Hayward N. J., Dean R. L., Harbeson S., Straub J. A., Li Z., Powers J. C. Calpain as a novel target for treating acute neurodegenerative disorders. Neurol Res. 1995 Aug;17(4):249–258. doi: 10.1080/01616412.1995.11740322. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996 Jul-Aug;9(5):836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Evans S. M., Hawkey C. J., Cole A. T., Balsitis M., Whittle B. J., Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993 Aug 7;342(8867):338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- Brown K., Park S., Kanno T., Franzoso G., Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford L. J., Tan B., McCarthy C. J., Hla T. Involvement of nuclear factor kappa B in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum. 1997 Feb;40(2):226–236. doi: 10.1002/art.1780400207. [DOI] [PubMed] [Google Scholar]
- Crow J. P., Beckman J. S. The role of peroxynitrite in nitric oxide-mediated toxicity. Curr Top Microbiol Immunol. 1995;196:57–73. doi: 10.1007/978-3-642-79130-7_7. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., Caputi A. P., Zingarelli B. Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology. 1998 Jan;93(1):96–101. doi: 10.1046/j.1365-2567.1998.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S., Zingarelli B., Costantino G., Szabó A., Salzman A. L., Caputi A. P., Szabó C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br J Pharmacol. 1997 Jul;121(6):1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S., Zingarelli B., Gilad E., Hake P., Salzman A. L., Szabó C. Protective effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthase in a carrageenan-induced model of local inflammation. Eur J Pharmacol. 1998 Jan 19;342(1):67–76. doi: 10.1016/s0014-2999(97)01417-9. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., Zingarelli B., Hake P., Salzman A. L., Szabó C. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic Biol Med. 1998 Feb;24(3):450–459. doi: 10.1016/s0891-5849(97)00280-3. [DOI] [PubMed] [Google Scholar]
- Eiserich J. P., Hristova M., Cross C. E., Jones A. D., Freeman B. A., Halliwell B., van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998 Jan 22;391(6665):393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- Futaki N., Arai I., Hamasaka Y., Takahashi S., Higuchi S., Otomo S. Selective inhibition of NS-398 on prostanoid production in inflamed tissue in rat carrageenan-air-pouch inflammation. J Pharm Pharmacol. 1993 Aug;45(8):753–755. doi: 10.1111/j.2042-7158.1993.tb07103.x. [DOI] [PubMed] [Google Scholar]
- Griscavage J. M., Wilk S., Ignarro L. J. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3308–3312. doi: 10.1073/pnas.93.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscavage J. M., Wilk S., Ignarro L. J. Serine and cysteine proteinase inhibitors prevent nitric oxide production by activated macrophages by interfering with transcription of the inducible NO synthase gene. Biochem Biophys Res Commun. 1995 Oct 13;215(2):721–729. doi: 10.1006/bbrc.1995.2523. [DOI] [PubMed] [Google Scholar]
- Grisham M. B. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994 Sep 24;344(8926):859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- Harada Y., Hatanaka K., Kawamura M., Saito M., Ogino M., Majima M., Ohno T., Ogino K., Yamamoto K., Taketani Y. Role of prostaglandin H synthase-2 in prostaglandin E2 formation in rat carrageenin-induced pleurisy. Prostaglandins. 1996 Jan;51(1):19–33. doi: 10.1016/0090-6980(95)00168-9. [DOI] [PubMed] [Google Scholar]
- Heller B., Wang Z. Q., Wagner E. F., Radons J., Bürkle A., Fehsel K., Burkart V., Kolb H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem. 1995 May 12;270(19):11176–11180. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- Henkel T., Machleidt T., Alkalay I., Krönke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993 Sep 9;365(6442):182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Ikeda I., Kasajima T., Ishiyama S., Shimojo T., Takeo Y., Nishikawa T., Kameoka S., Hiroe M., Mitsunaga A. Distribution of inducible nitric oxide synthase in ulcerative colitis. Am J Gastroenterol. 1997 Aug;92(8):1339–1341. [PubMed] [Google Scholar]
- Inoue S., Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995 Aug 28;371(1):86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- Iwamoto H., Miura T., Okamura T., Shirakawa K., Iwatate M., Kawamura S., Tatsuno H., Ikeda Y., Matsuzaki M. Calpain inhibitor-1 reduces infarct size and DNA fragmentation of myocardium in ischemic/reperfused rat heart. J Cardiovasc Pharmacol. 1999 Apr;33(4):580–586. doi: 10.1097/00005344-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Kampfl A., Posmantur R. M., Zhao X., Schmutzhard E., Clifton G. L., Hayes R. L. Mechanisms of calpain proteolysis following traumatic brain injury: implications for pathology and therapy: implications for pathology and therapy: a review and update. J Neurotrauma. 1997 Mar;14(3):121–134. doi: 10.1089/neu.1997.14.121. [DOI] [PubMed] [Google Scholar]
- Kengatharan M., De Kimpe S. J., Thiemermann C. Analysis of the signal transduction in the induction of nitric oxide synthase by lipoteichoic acid in macrophages. Br J Pharmacol. 1996 Mar;117(6):1163–1170. doi: 10.1111/j.1476-5381.1996.tb16711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahora T., Suzuki K., Asakura H., Yoshida T., Suematsu M., Watanabe M., Aiso S., Tsuchiya M. Active oxygen species generated by monocytes and polymorphonuclear cells in Crohn's disease. Dig Dis Sci. 1988 Aug;33(8):951–955. doi: 10.1007/BF01535990. [DOI] [PubMed] [Google Scholar]
- Kohli V., Gao W., Camargo C. A., Jr, Clavien P. A. Calpain is a mediator of preservation-reperfusion injury in rat liver transplantation. Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):9354–9359. doi: 10.1073/pnas.94.17.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli V., Madden J. F., Bentley R. C., Clavien P. A. Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology. 1999 Jan;116(1):168–178. doi: 10.1016/s0016-5085(99)70241-6. [DOI] [PubMed] [Google Scholar]
- Kohli V., Madden J. F., Bentley R. C., Clavien P. A. Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology. 1999 Jan;116(1):168–178. doi: 10.1016/s0016-5085(99)70241-6. [DOI] [PubMed] [Google Scholar]
- Lih-Brody L., Powell S. R., Collier K. P., Reddy G. M., Cerchia R., Kahn E., Weissman G. S., Katz S., Floyd R. A., McKinley M. J. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996 Oct;41(10):2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- Liu Z. Q., Kunimatsu M., Yang J. P., Ozaki Y., Sasaki M., Okamoto T. Proteolytic processing of nuclear factor kappa B by calpain in vitro. FEBS Lett. 1996 Apr 29;385(1-2):109–113. doi: 10.1016/0014-5793(96)00360-2. [DOI] [PubMed] [Google Scholar]
- Lundberg J. O., Hellström P. M., Lundberg J. M., Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994 Dec 17;344(8938):1673–1674. doi: 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Markgraf C. G., Velayo N. L., Johnson M. P., McCarty D. R., Medhi S., Koehl J. R., Chmielewski P. A., Linnik M. D. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke. 1998 Jan;29(1):152–158. doi: 10.1161/01.str.29.1.152. [DOI] [PubMed] [Google Scholar]
- Markgraf C. G., Velayo N. L., Johnson M. P., McCarty D. R., Medhi S., Koehl J. R., Chmielewski P. A., Linnik M. D. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke. 1998 Jan;29(1):152–158. doi: 10.1161/01.str.29.1.152. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Kusuoka H., Inoue M., Hori M., Kamada T. Protective effect of the protease inhibitor leupeptin against myocardial stunning. J Cardiovasc Pharmacol. 1993 Jul;22(1):135–142. doi: 10.1097/00005344-199307000-00021. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S. The calpains. Trends Neurosci. 1989 Nov;12(11):438–444. doi: 10.1016/0166-2236(89)90093-3. [DOI] [PubMed] [Google Scholar]
- Middleton S. J., Shorthouse M., Hunter J. O. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993 Feb 20;341(8843):465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Thompson J. H., Zhang X. J., Sadowska-Krowicka H., Kakkis J. L., Munshi U. K., Sandoval M., Rossi J. L., Eloby-Childress S., Beckman J. S. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995 Nov;109(5):1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Thompson J. H., Zhang X. J., Sadowska-Krowicka H., Kakkis J. L., Munshi U. K., Sandoval M., Rossi J. L., Eloby-Childress S., Beckman J. S. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995 Nov;109(5):1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Thompson J. H., Zhang X. J., Sadowska-Krowicka H., Kakkis J. L., Munshi U. K., Sandoval M., Rossi J. L., Eloby-Childress S., Beckman J. S. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995 Nov;109(5):1475–1483. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- Milligan S. A., Owens M. W., Grisham M. B. Inhibition of IkappaB-alpha and IkappaB-beta proteolysis by calpain inhibitor I blocks nitric oxide synthesis. Arch Biochem Biophys. 1996 Nov 15;335(2):388–395. doi: 10.1006/abbi.1996.9998. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Mourelle M., Vilaseca J., Guarner F., Salas A., Malagelada J. R. Toxic dilatation of colon in a rat model of colitis is linked to an inducible form of nitric oxide synthase. Am J Physiol. 1996 Mar;270(3 Pt 1):G425–G430. doi: 10.1152/ajpgi.1996.270.3.G425. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Karmeli F., Okon E. Sulfhydryl blocker-induced rat colonic inflammation is ameliorated by inhibition of nitric oxide synthase. Gastroenterology. 1995 Jul;109(1):98–106. doi: 10.1016/0016-5085(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Stamler J. S., Karmeli F., Mullins M. E., Singel D. J., Loscalzo J., Xavier R. J., Podolsky D. K. Peroxynitrite-induced rat colitis--a new model of colonic inflammation. Gastroenterology. 1993 Dec;105(6):1681–1688. doi: 10.1016/0016-5085(93)91063-n. [DOI] [PubMed] [Google Scholar]
- Rami A., Krieglstein J. Protective effects of calpain inhibitors against neuronal damage caused by cytotoxic hypoxia in vitro and ischemia in vivo. Brain Res. 1993 Apr 23;609(1-2):67–70. doi: 10.1016/0006-8993(93)90856-i. [DOI] [PubMed] [Google Scholar]
- Ruetten H., Thiemermann C. Effect of calpain inhibitor I, an inhibitor of the proteolysis of I kappa B, on the circulatory failure and multiple organ dysfunction caused by endotoxin in the rat. Br J Pharmacol. 1997 Jun;121(4):695–704. doi: 10.1038/sj.bjp.0701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido T. C., Sorimachi H., Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994 Aug;8(11):814–822. [PubMed] [Google Scholar]
- Salgo M. G., Bermúdez E., Squadrito G. L., Pryor W. A. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes [corrected]. Arch Biochem Biophys. 1995 Oct 1;322(2):500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- Salzman A. L. Nitric oxide in the gut. New Horiz. 1995 Feb;3(1):33–45. [PubMed] [Google Scholar]
- Sautebin L., Ialenti A., Ianaro A., Di Rosa M. Relationship between nitric oxide and prostaglandins in carrageenin pleurisy. Biochem Pharmacol. 1998 Apr 1;55(7):1113–1117. doi: 10.1016/s0006-2952(97)00530-3. [DOI] [PubMed] [Google Scholar]
- Shiratora Y., Aoki S., Takada H., Kiriyama H., Ohto K., Hai K., Teraoka H., Matano S., Matsumoto K., Kamii K. Oxygen-derived free radical generating capacity of polymorphonuclear cells in patients with ulcerative colitis. Digestion. 1989;44(3):163–171. doi: 10.1159/000199906. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R., Van Someren R. N., Blake D. R., Rampton D. S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992 Jul;103(1):186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- Sorimachi Y., Harada K., Saido T. C., Ono T., Kawashima S., Yoshida K. Downregulation of calpastatin in rat heart after brief ischemia and reperfusion. J Biochem. 1997 Oct;122(4):743–748. doi: 10.1093/oxfordjournals.jbchem.a021818. [DOI] [PubMed] [Google Scholar]
- Szabó C., Lim L. H., Cuzzocrea S., Getting S. J., Zingarelli B., Flower R. J., Salzman A. L., Perretti M. Inhibition of poly (ADP-ribose) synthetase attenuates neutrophil recruitment and exerts antiinflammatory effects. J Exp Med. 1997 Oct 6;186(7):1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996 Aug;6(2):79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Szabó C., Virág L., Cuzzocrea S., Scott G. S., Hake P., O'Connor M. P., Zingarelli B., Salzman A., Kun E. Protection against peroxynitrite-induced fibroblast injury and arthritis development by inhibition of poly(ADP-ribose) synthase. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3867–3872. doi: 10.1073/pnas.95.7.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Bowes J., Myint F. P., Vane J. R. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci U S A. 1997 Jan 21;94(2):679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda G., Matsushita S., Kuramoto K., Oda S., Ezaki H., Hattori A., Kawashima S. Calcium-activated neutral protease inhibitor (E-64c) and reperfusion for experimental myocardial infarction. Jpn Heart J. 1989 May;30(3):375–386. doi: 10.1536/ihj.30.375. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Gale D., Shoupe T. S. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology. 1992 Jan;102(1):18–27. doi: 10.1016/0016-5085(92)91779-4. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Nath R., Posner A., Raser K. J., Buroker-Kilgore M., Hajimohammadreza I., Probert A W., Jr, Marcoux F. W., Ye Q., Takano E. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. K., Nath R., Posner A., Raser K. J., Buroker-Kilgore M., Hajimohammadreza I., Probert A W., Jr, Marcoux F. W., Ye Q., Takano E. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. K., Yuen P. W. Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol Sci. 1994 Nov;15(11):412–419. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Wiseman H., Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996 Jan 1;313(Pt 1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994 Feb 18;269(7):4705–4708. [PubMed] [Google Scholar]
- Yamamoto K., Arakawa T., Ueda N., Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995 Dec 29;270(52):31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Inui M., Harada K., Saido T. C., Sorimachi Y., Ishihara T., Kawashima S., Sobue K. Reperfusion of rat heart after brief ischemia induces proteolysis of calspectin (nonerythroid spectrin or fodrin) by calpain. Circ Res. 1995 Sep;77(3):603–610. doi: 10.1161/01.res.77.3.603. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Inui M., Harada K., Saido T. C., Sorimachi Y., Ishihara T., Kawashima S., Sobue K. Reperfusion of rat heart after brief ischemia induces proteolysis of calspectin (nonerythroid spectrin or fodrin) by calpain. Circ Res. 1995 Sep;77(3):603–610. doi: 10.1161/01.res.77.3.603. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Yamasaki Y., Kawashima S. Calpain activity alters in rat myocardial subfractions after ischemia or reperfusion. Biochim Biophys Acta. 1993 Sep 8;1182(2):215–220. doi: 10.1016/0925-4439(93)90143-o. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Yamasaki Y., Kawashima S. Calpain activity alters in rat myocardial subfractions after ischemia or reperfusion. Biochim Biophys Acta. 1993 Sep 8;1182(2):215–220. doi: 10.1016/0925-4439(93)90143-o. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., Cuzzocrea S., Szabó C., Salzman A. L. Mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1998 Dec;287(3):1048–1055. [PubMed] [Google Scholar]
- Zingarelli B., Cuzzocrea S., Zsengellér Z., Salzman A. L., Szabó C. Protection against myocardial ischemia and reperfusion injury by 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase. Cardiovasc Res. 1997 Nov;36(2):205–215. doi: 10.1016/s0008-6363(97)00137-5. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., O'Connor M., Wong H., Salzman A. L., Szabó C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996 Jan 1;156(1):350–358. [PubMed] [Google Scholar]
- Zingarelli B., Squadrito F., Graziani P., Camerini R., Caputi A. P. Effects of zileuton, a new 5-lipoxygenase inhibitor, in experimentally induced colitis in rats. Agents Actions. 1993 Jul;39(3-4):150–156. doi: 10.1007/BF01998968. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., Szabó C., Salzman A. L. Blockade of Poly(ADP-ribose) synthetase inhibits neutrophil recruitment, oxidant generation, and mucosal injury in murine colitis. Gastroenterology. 1999 Feb;116(2):335–345. doi: 10.1016/s0016-5085(99)70130-7. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., Szabó C., Salzman A. L. Reduced oxidative and nitrosative damage in murine experimental colitis in the absence of inducible nitric oxide synthase. Gut. 1999 Aug;45(2):199–209. doi: 10.1136/gut.45.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]