Abstract
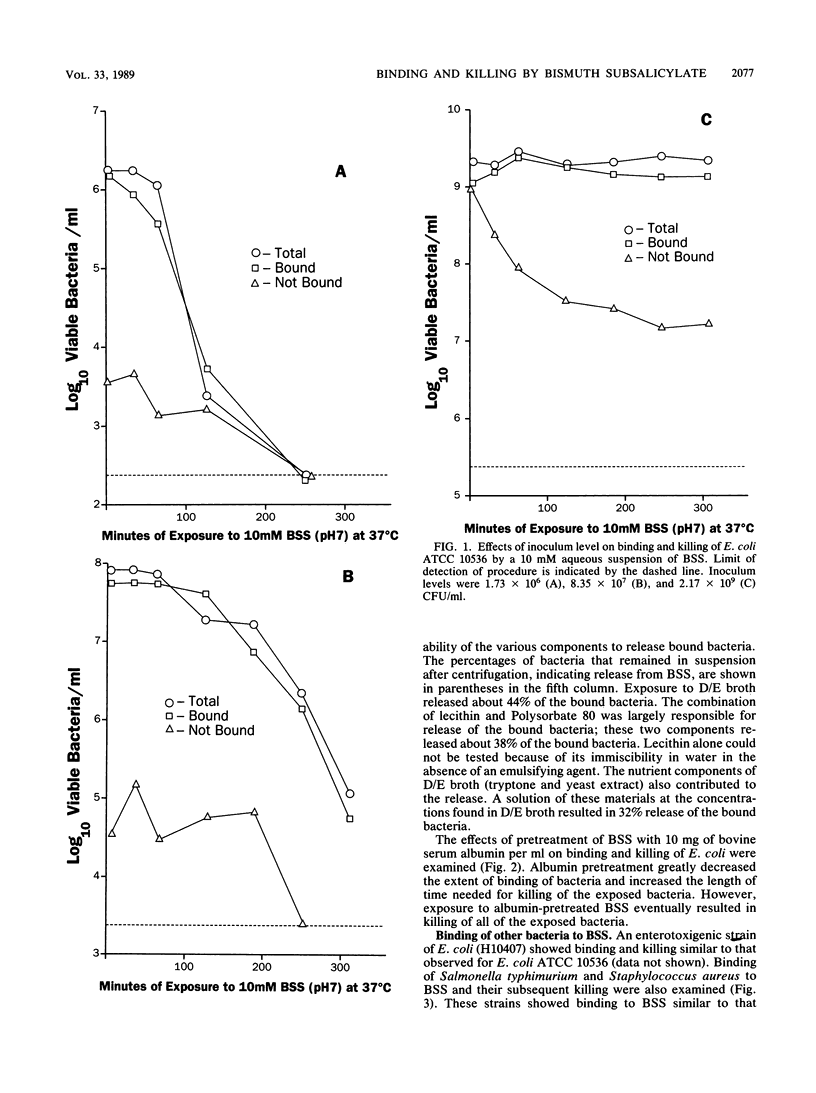
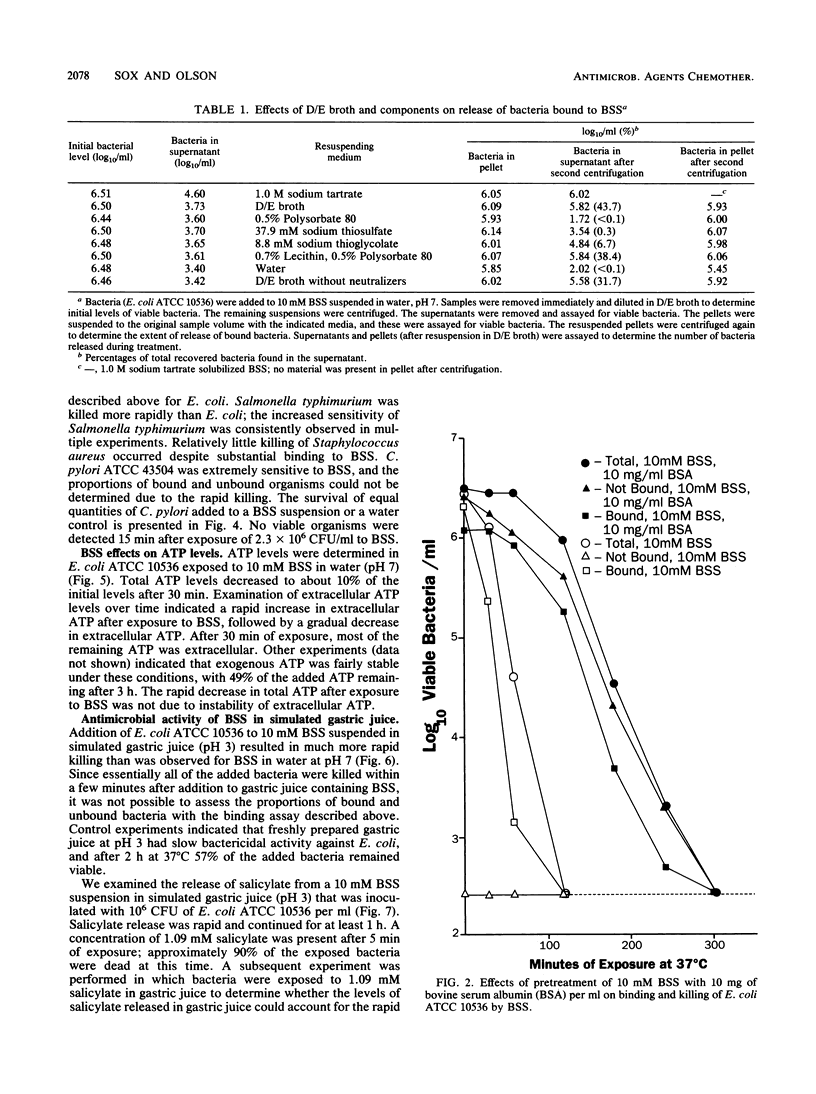
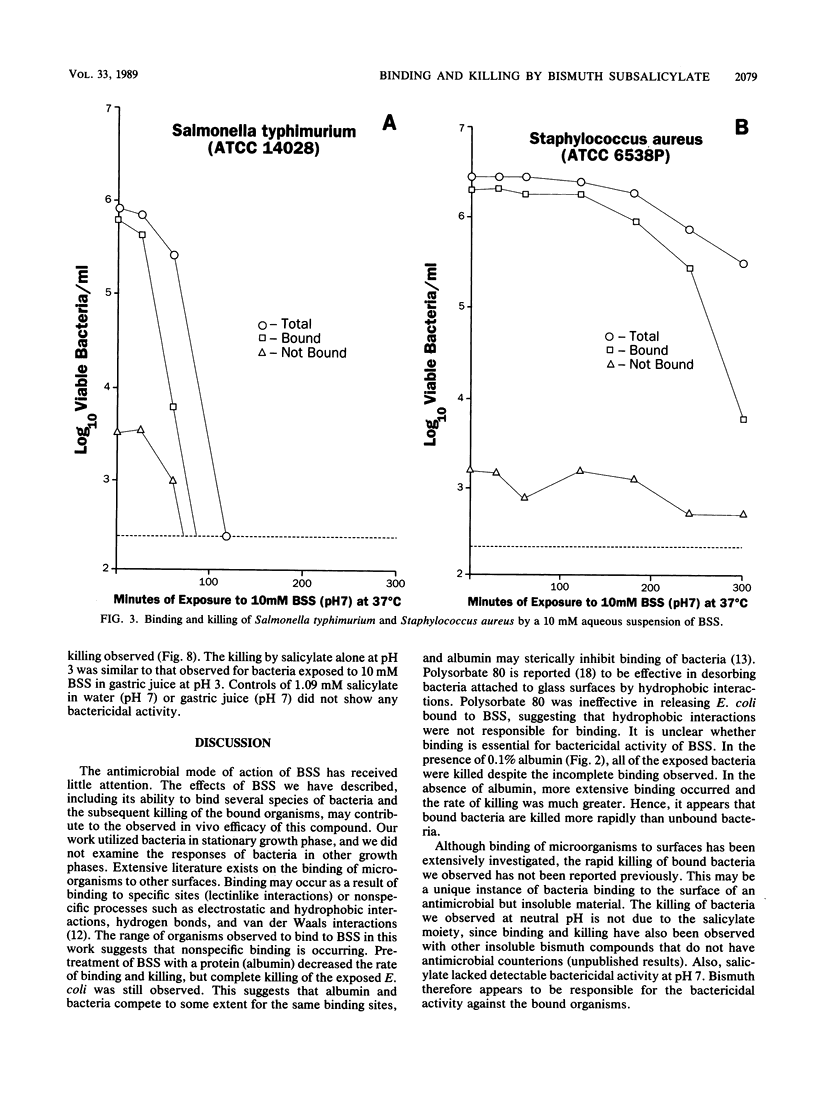
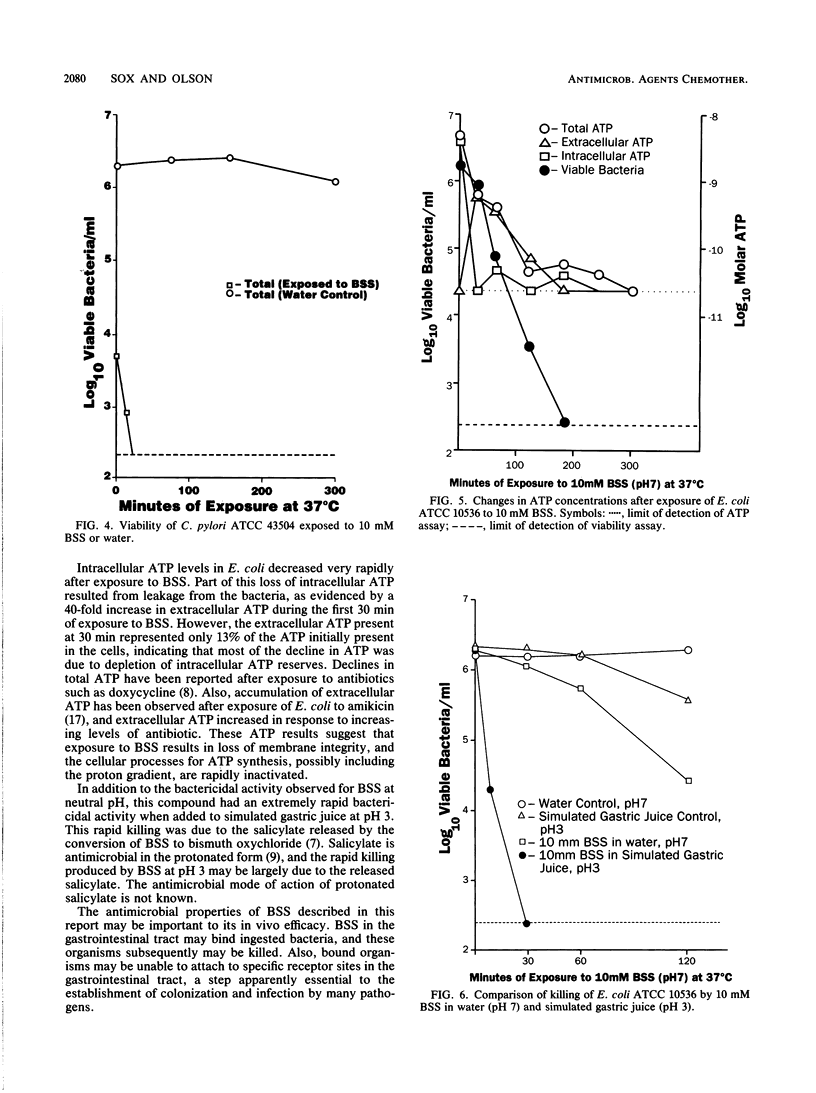
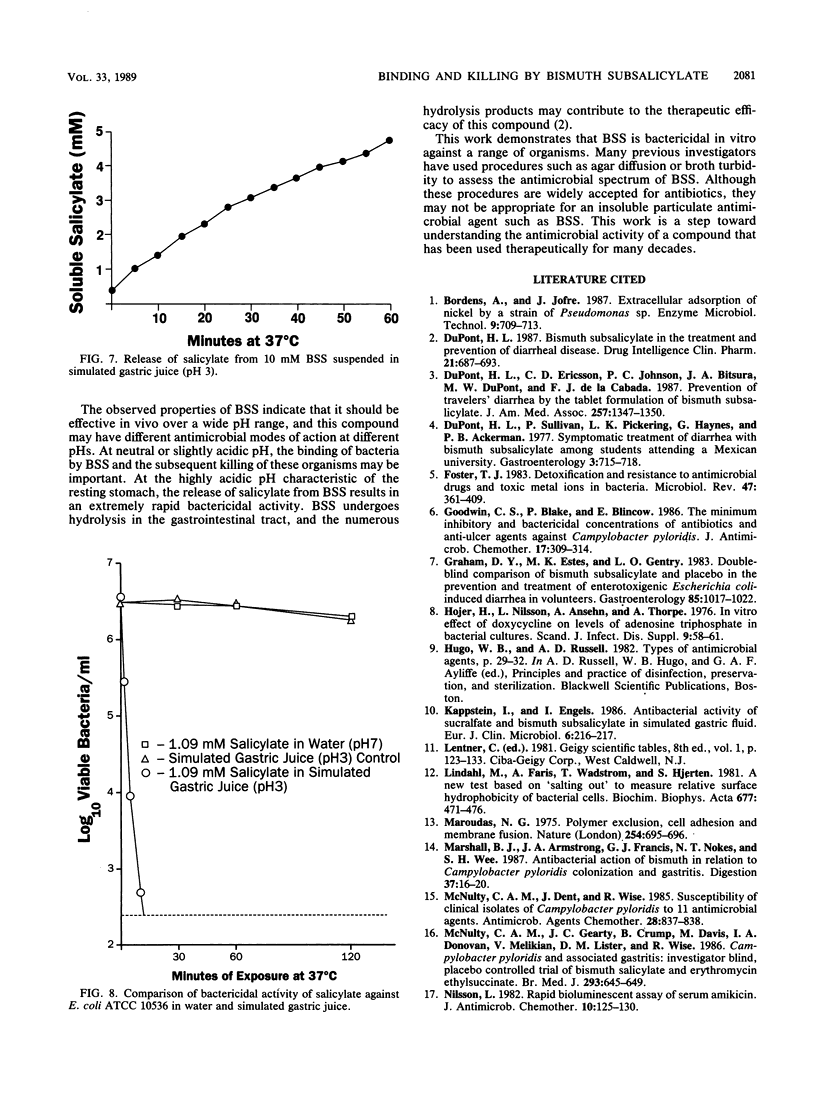
Bismuth subsalicylate (BSS) is a compound without significant aqueous solubility that is widely used for the treatment of gastrointestinal disorders. BSS was able to bind bacteria of diverse species, and these bound bacteria were subsequently killed. A 4-log10 reduction of viable bacteria occurred within 4 h after a 10 mM aqueous suspension of BSS was inoculated with 2 x 10(6) Escherichia coli cells per ml. Binding and killing were dependent on the levels of inoculated bacteria, and significant binding but little killing of the exposed bacteria occurred at an inoculum level of 2 x 10(9) E. coli per ml. Intracellular ATP decreased rapidly after exposure of E. coli to 10 mM BSS and, after 30 min, was only 1% of the original level. Extracellular ATP increased after exposure to BSS, but the accumulation of extracellular ATP was not sufficient to account for the loss of intracellular ATP. The killing of bacteria exposed to BSS may have been due to cessation of ATP synthesis or a loss of membrane integrity. Bactericidal activity of BSS was also investigated in a simulated gastric juice at pH 3. Killing of E. coli at this pH was much more rapid than at pH 7 and was apparently due to salicylate released by the conversion of BSS to bismuth oxychloride. It is proposed that the binding and killing observed for BSS contribute to the efficacy of this compound against gastrointestinal infections such as traveler's diarrhea.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DuPont H. L. Bismuth subsalicylate in the treatment and prevention of diarrheal disease. Drug Intell Clin Pharm. 1987 Sep;21(9):687–693. doi: 10.1177/106002808702100901. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Ericsson C. D., Johnson P. C., Bitsura J. A., DuPont M. W., de la Cabada F. J. Prevention of travelers' diarrhea by the tablet formulation of bismuth subsalicylate. JAMA. 1987 Mar 13;257(10):1347–1350. [PubMed] [Google Scholar]
- DuPont H. L., Sullivan P., Pickering L. K., Haynes G., Ackerman P. B. Symptomatic treatment of diarrhea with bismuth subsalicylate among students attending a Mexican university. Gastroenterology. 1977 Oct;73(4 Pt 1):715–718. [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S., Blake P., Blincow E. The minimum inhibitory and bactericidal concentrations of antibiotics and anti-ulcer agents against Campylobacter pyloridis. J Antimicrob Chemother. 1986 Mar;17(3):309–314. doi: 10.1093/jac/17.3.309. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K., Gentry L. O. Double-blind comparison of bismuth subsalicylate and placebo in the prevention and treatment of enterotoxigenic Escherichia coli-induced diarrhea in volunteers. Gastroenterology. 1983 Nov;85(5):1017–1022. [PubMed] [Google Scholar]
- Höjer H., Nilsson L., Anséhn S., Thore A. In-vitro effect of doxycycline on levels of adenosine triphosphate in bacterial cultures. Possible clinical applications. Scand J Infect Dis Suppl. 1976;(9):58–61. [PubMed] [Google Scholar]
- Kappstein I., Engels I. Antibacterial activity of sucralfate and bismuth subsalicylate in simulated gastric fluid. Eur J Clin Microbiol. 1987 Apr;6(2):216–217. doi: 10.1007/BF02018225. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Maroudas N. G. Polymer exclusion, cell adhesion and membrane fusion. Nature. 1975 Apr 24;254(5502):695–696. doi: 10.1038/254695a0. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Armstrong J. A., Francis G. J., Nokes N. T., Wee S. H. Antibacterial action of bismuth in relation to Campylobacter pyloridis colonization and gastritis. Digestion. 1987;37 (Suppl 2):16–30. doi: 10.1159/000199555. [DOI] [PubMed] [Google Scholar]
- McNulty C. A., Dent J., Wise R. Susceptibility of clinical isolates of Campylobacter pyloridis to 11 antimicrobial agents. Antimicrob Agents Chemother. 1985 Dec;28(6):837–838. doi: 10.1128/aac.28.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty C. A., Gearty J. C., Crump B., Davis M., Donovan I. A., Melikian V., Lister D. M., Wise R. Campylobacter pyloridis and associated gastritis: investigator blind, placebo controlled trial of bismuth salicylate and erythromycin ethylsuccinate. Br Med J (Clin Res Ed) 1986 Sep 13;293(6548):645–649. doi: 10.1136/bmj.293.6548.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L. Rapid bioluminescent assay of serum amikacin. J Antimicrob Chemother. 1982 Aug;10(2):125–130. doi: 10.1093/jac/10.2.125. [DOI] [PubMed] [Google Scholar]
- Orstavik D. Sorption of Streptococcus faecium to glass. Acta Pathol Microbiol Scand B. 1977 Feb;85B(1):38–46. [PubMed] [Google Scholar]
- Rauws E. A., Tytgat G. N. Elektronenmikroskopische Befunde während einer Behandlung campylobacter-pylori-positiver Gastritiden mit Wismutsalzen. Z Gastroenterol. 1987 Sep;25 (Suppl 4):41–43. [PubMed] [Google Scholar]
- Strandberg G. W., Shumate S. E., Parrott J. R. Microbial Cells as Biosorbents for Heavy Metals: Accumulation of Uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa. Appl Environ Microbiol. 1981 Jan;41(1):237–245. doi: 10.1128/aem.41.1.237-245.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore A., Nilsson L., Höjer H., Anséhn S., Bröte L. Effects of ampicillin on intracellular levels of adenosine triphosphate in bacterial cultures related to antibiotic susceptibility. Acta Pathol Microbiol Scand B. 1977 Apr;85(2):161–166. doi: 10.1111/j.1699-0463.1977.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Edwards H. W. Microbial uptake of lead. Science. 1972 Jun 23;176(4041):1334–1335. doi: 10.1126/science.176.4041.1334. [DOI] [PubMed] [Google Scholar]


