Abstract
BACKGROUND—In a previous study we showed that interleukin 1β (IL-1β) caused recurrence of gastric ulcers in rats, and that adhesion molecules (intercellular adhesion molecule 1 and leucocytic β2 integrins) play a role in this recurrence. Although gastric acid plays an important role in many types of gastric injuries, including peptic ulcer recurrence, the mechanism(s) remains unclear. AIMS—To examine the involvement of gastric acid in induction of ulcer recurrence by IL-1β, and to investigate the role of gastric acid in inflammatory responses during ulcer recurrence. METHODS—Rats with healed ulcers were used. Rats were given 1 µg/kg IL-1β intraperitoneally. Another group of rats was given 20 mg/kg omeprazole for three days to inhibit acid secretion, and received IL-1β 20 hours after the first administration of omeprazole. They were then given 0.15 N HCl or vehicle at 0, 12, 24, and 36 hours after IL-1β treatment. Some rats were given acid alone at the same time points. Expression of adhesion molecules was examined immunohistochemically and concentrations of IL-1β and tumour necrosis factor α (TNF-α) were measured by ELISA in scar tissue 24 hours after IL-1β treatment. RESULTS—IL-1β increased expression of adhesion molecules and concentrations of IL-1β and TNF-α in scar tissue by 24 hours after IL-1β treatment, and nine of 11 healed ulcers had recurred by 48 hours. Omeprazole inhibited the effects of IL-1β. HCl acid abolished the inhibitory effects of omeprazole. Acid alone affected neither expression of adhesion molecules nor cytokine concentrations, and did not cause recurrence. CONCLUSIONS—Gastric acid is required for recurrence of gastric ulcers caused by IL-1β, and gastric acid stimulates the inflammatory process in scarred mucosa during ulcer recurrence. Keywords: ulcer recurrence; gastric acid; inflammatory cytokines; adhesion molecules; inflammation
Full Text
The Full Text of this article is available as a PDF (228.1 KB).
Figure 1 .
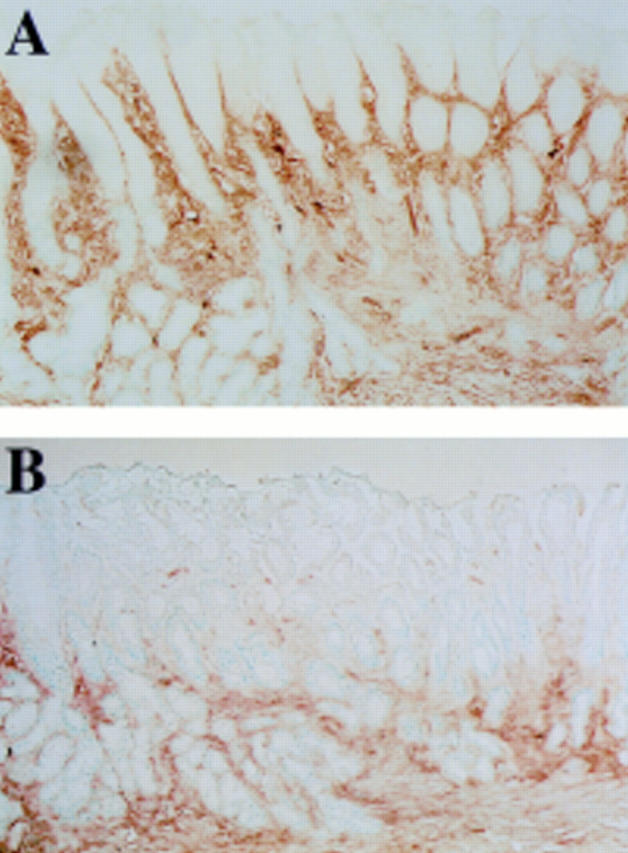
Expression of intercellular adhesion molecule 1 (ICAM-1) in scarred mucosa 24 hours after injection of interleukin 1β (IL-1β). A marked increase in expression of ICAM-1 was observed by 24 hours in rats given IL-1β (A) while there was no increase in expression of ICAM-1 in rats administered omeprazole together with IL-1β (B). Original magnification ×40.
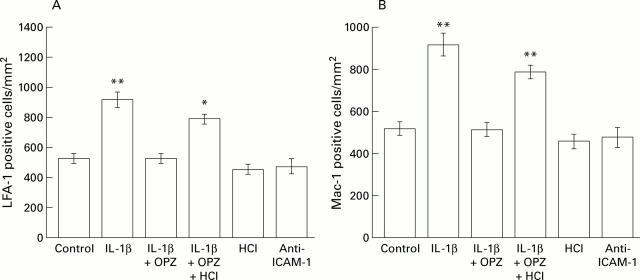
Figure 2 .
Comparison of numbers of infiltrating leucocytes positive for lymphocyte function associated antigen 1 (LFA-1) or Mac-1 in scarred mucosa. The numbers of LFA-1 or Mac-1 positive cells infiltrating scarred mucosa were counted, and the mean numbers of such cells were compared with those in control animals (not given IL-1β). Results are mean (SEM), n=6. OPZ, omeprazole; IL-1β, interleukin 1β; ICAM-1, intercellular adhesion molecule 1. *p<0.05, **p<0.01 compared with control animals.
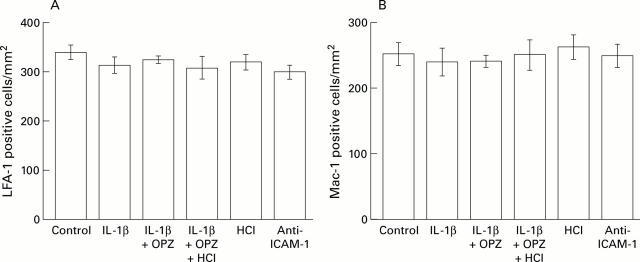
Figure 3 .
Comparison of numbers of infiltrating leucocytes positive for lymphocyte function associated antigen 1 (LFA-1) or Mac-1 in normal mucosa. The numbers of LFA-1 or Mac-1 positive cells infiltrating the normal mucosa were counted, and the mean numbers of such cells were compared with those in control animals (not given IL-1β). Results are mean (SEM), n=6. OPZ, omeprazole; IL-1β, interleukin 1β; ICAM-1, intercellular adhesion molecule 1.
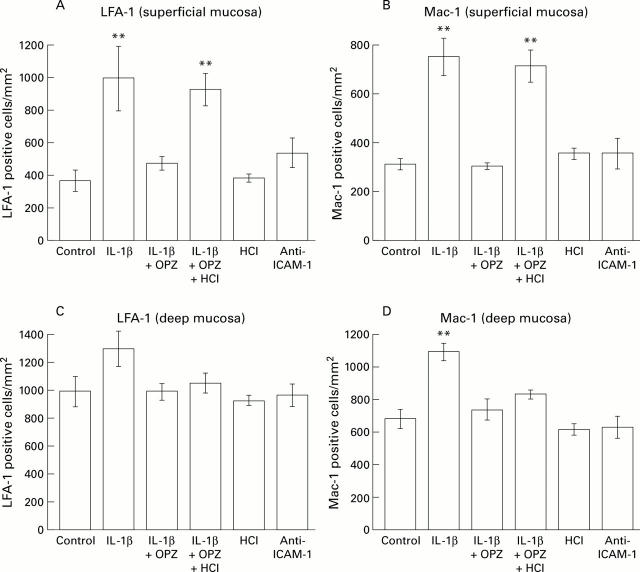
Figure 4 .
Comparison of numbers of lymphocyte function associated antigen 1 (LFA-1) and Mac-1 positive leucocytes in superficial and deep portions of scarred mucosa at 24 hours. LFA-1 and Mac-1 positive cells were counted separately in the deep and superficial portions of scarred mucosa, and the mean numbers of cells were compared with those in control animals (not given IL-1β). Results are mean (SEM), n=6. OPZ, omeprazole; IL-1β, interleukin 1β; ICAM-1, intercellular adhesion molecule 1. **p<0.01 compared with control animals.
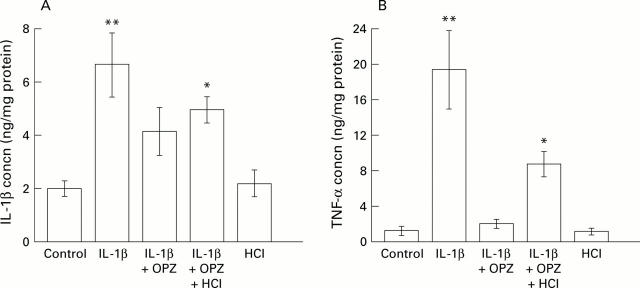
Figure 5 .
Interleukin 1β (IL-1β) and tumour necrosis factor α (TNF-α) concentrations in scar tissue at 24 hours. Concentrations of IL-1β (A) and TNF-α (B) in scar tissue were assayed 24 hours after IL-1β treatment. Results are mean (SEM), n=6. OPZ, omeprazole. *p<0.05, **p<0.01 compared with control animals (not given IL-1β).
Figure 6 .
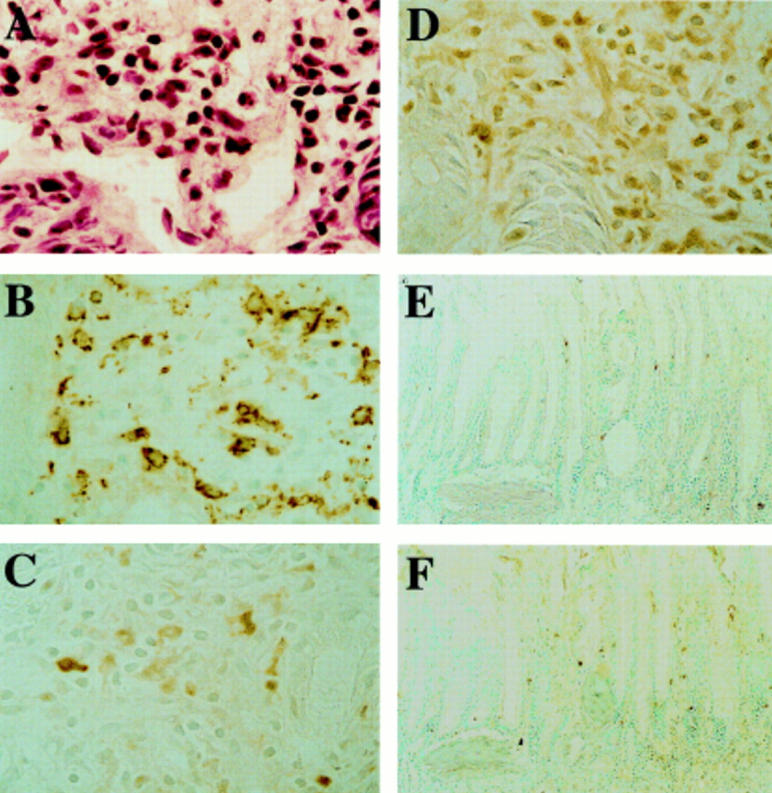
Cellular infiltration and immunohistochemical staining for interleukin 1β (IL-1β) and tumour necrosis factor α (TNF-α) in superficial mucosa at 24 hours after IL-1β treatment. (A) IL-1β induced infiltration by leucocytes, including neutrophils, in the superficial portion of scarred mucosa. (B) Monocytes/macrophages were abundant in this region. IL-1β (C) and TNF-α (D) were detected mainly in inflammatory cells in the superficial mucosa. The numbers of cells stained for IL-1β (E) and TNF-α (F) were small in rats given the antibody against intercellular adhesion molecule 1. Original magnification ×200 (A-D) and ×50 (E, F).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews F. J., Malcontenti-Wilson C., O'Brien P. E. Effect of nonsteroidal anti-inflammatory drugs on LFA-1 and ICAM-1 expression in gastric mucosa. Am J Physiol. 1994 Apr;266(4 Pt 1):G657–G664. doi: 10.1152/ajpgi.1994.266.4.G657. [DOI] [PubMed] [Google Scholar]
- Brenner A. J., Harris E. D. A quantitative test for copper using bicinchoninic acid. Anal Biochem. 1995 Mar 20;226(1):80–84. doi: 10.1006/abio.1995.1194. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Pesek I. Glomerular tumor necrosis factor and interleukin 1 during acute aminonucleoside nephrosis. An immunohistochemical study. Lab Invest. 1991 Jan;64(1):21–28. [PubMed] [Google Scholar]
- Fukawa K., Kawano O., Misaki N., Uchida M., Irino O. Experimental studies on gastric ulcer. (4). Sequential observation and evaluation of gastric ulcers by endoscope in the rat. Jpn J Pharmacol. 1983 Feb;33(1):175–179. doi: 10.1254/jjp.33.175. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Lew G. M., Klein P. D., Evans D. G., Evans D. J., Jr, Saeed Z. A., Malaty H. M. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992 May 1;116(9):705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- Gudmand-Høyer E., Jensen K. B., Krag E., Rask-Madsen J., Rahbek I., Rune S. J., Wulff H. R. Prophylactic effect of cimetidine in duodenal ulcer disease. Br Med J. 1978 Apr 29;1(6120):1095–1097. doi: 10.1136/bmj.1.6120.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvietys P. R., Twohig B., Danzell J., Specian R. D. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology. 1990 Apr;98(4):909–920. doi: 10.1016/0016-5085(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Lauritsen K., Andersen B. N., Laursen L. S., Hansen J., Havelund T., Eriksen J., Rehfeld J. F., Kjaergaard J., Rask-Madsen J. Omeprazole 20 mg three days a week and 10 mg daily in prevention of duodenal ulcer relapse. Double-blind comparative trial. Gastroenterology. 1991 Mar;100(3):663–669. doi: 10.1016/0016-5085(91)80009-x. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Marshall B. J., Goodwin C. S., Warren J. R., Murray R., Blincow E. D., Blackbourn S. J., Phillips M., Waters T. E., Sanderson C. R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988 Dec 24;2(8626-8627):1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Ward P. A. Immune complex-induced lung and dermal vascular injury. Differing requirements for tumor necrosis factor-alpha and IL-1. J Immunol. 1992 Jul 1;149(1):331–339. [PubMed] [Google Scholar]
- Noach L. A., Bosma N. B., Jansen J., Hoek F. J., van Deventer S. J., Tytgat G. N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994 May;29(5):425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Robert A., Olafsson A. S., Lancaster C., Zhang W. R. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, and retards gastric emptying. Life Sci. 1991;48(2):123–134. doi: 10.1016/0024-3205(91)90405-z. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Arondel J., Cavaillon J. M., Huerre M. Role of interleukin-1 in the pathogenesis of experimental shigellosis. J Clin Invest. 1995 Aug;96(2):884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci L., Fiorucci S., Di Matteo F. M., Morelli A. Role of tumor necrosis factor alpha release and leukocyte margination in indomethacin-induced gastric injury in rats. Gastroenterology. 1995 Feb;108(2):393–401. doi: 10.1016/0016-5085(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Saperas E., Taché Y. Central interleukin-1 beta-induced inhibition of acid secretion in rats: specificity of action. Life Sci. 1993;52(9):785–792. doi: 10.1016/0024-3205(93)90076-f. [DOI] [PubMed] [Google Scholar]
- Segawa K., Nakazawa S., Tsukamoto Y., Chujoh C., Yamao K., Hase S. Effect of omeprazole on gastric acid secretion in rat: evaluation of dose, duration of effect, and route of administration. Gastroenterol Jpn. 1987 Aug;22(4):413–418. doi: 10.1007/BF02773807. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Mori M., Miura S., Suematsu M., Fukumura D., Kimura H., Ishii H. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radic Biol Med. 1996;21(5):727–731. doi: 10.1016/0891-5849(96)00180-3. [DOI] [PubMed] [Google Scholar]
- Takagi K., Okabe S., Saziki R. A new method for the production of chronic gastric ulcer in rats and the effect of several drugs on its healing. Jpn J Pharmacol. 1969 Sep;19(3):418–426. doi: 10.1254/jjp.19.418. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- Uehara A., Okumura T., Sekiya C., Okamura K., Takasugi Y., Namiki M. Interleukin-1 inhibits the secretion of gastric acid in rats: possible involvement of prostaglandin. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1578–1584. doi: 10.1016/0006-291x(89)90855-3. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Arfors K. E., McKnight G. W. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991 Apr;100(4):878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Wandall J. H. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. 1992 May;33(5):617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Arakawa T., Fukuda T., Higuchi K., Kobayashi K. Role of neutrophils in a rat model of gastric ulcer recurrence caused by interleukin-1 beta. Am J Pathol. 1997 Mar;150(3):971–979. [PMC free article] [PubMed] [Google Scholar]