Abstract
BACKGROUND AND AIMS—Helicobacter pylori infection induces expression of proinflammatory cytokines such as interleukin (IL)-8 and tumour necrosis factor α (TNF-α) in gastric mucosa, and their genes have AP-1 binding sites in the promoter region. c-Fos is important for transactivation of AP-1 which has SRE in the promoter region. We conducted this study to confirm H pylori induced transactivation of these binding sites. METHODS—Transactivation of SRE and AP-1 was evaluated in human gastric cancer cells TMK1 and MKN45 by luciferase reporter assay in transient transfection. We compared the effects of coculture with four H pylori strains, a cag pathogenicity island (PAI) positive strain TN2, its isogenic vacA negative (TN2-ΔvacA) or cagE negative (TN2-ΔcagE) mutants, and a cag PAI negative clinical isolate T68. Phosphorylation of ERK1/2, JNK, and c-Jun was measured by immunoblot, induction of IL-8 secretion by ELISA, and the effects of MEK by inhibitor U0126. RESULTS—Both SRE and AP-1 were transactivated by coculture with TN2. Although TN2-ΔvacA induced comparable transactivation, TN2-ΔcagE and T68 showed decreased transactivation of SRE (65% and 51%) and AP-1 (71% and 54%, respectively, of TN2). Heat killed TN2 or indirect contact using a permeable membrane inhibited transactivation. Levels of phosphorylated ERK1/2, JNK, and c-Jun were increased by coculture with TN2. MEK inhibitor U0126 reduced TN2 induced transactivation of SRE and AP1, as well as secretion of IL-8, by 83%, 87%, and 53%, respectively, of TN2. CONCLUSIONS—Transactivation of SRE and AP-1, through ERK/MAPK and JNK/SAPK cascades, respectively, was found in gastric cancer cells cocultured with H pylori. Direct contact with viable bacteria possessing intact cag PAI is a prerequisite for the onset of intracellular signalling leading to AP-1 transactivation. Keywords: Helicobacter pylori; SRE; AP-1; cag pathogenicity island PAI; gastric cancer
Full Text
The Full Text of this article is available as a PDF (154.1 KB).
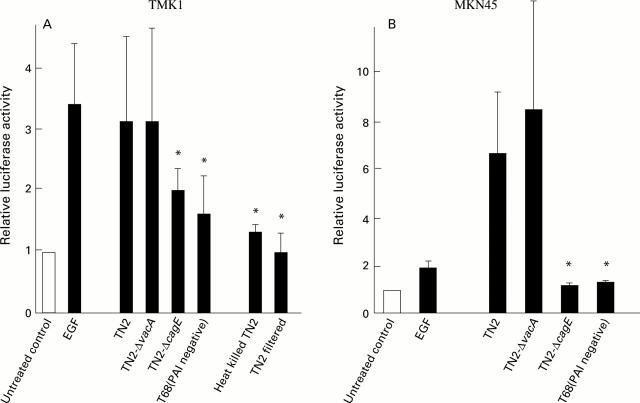
Figure 1 .
Transactivation of SRE by Helicobacter pylori. Transactivation of SRE in TMK1 (A) and MKN45 (B) cells induced by H pylori was measured by luciferase assay using pSRE-Luc. The TN2 strain was positive for two known virulence factors, CagA and VacA, and had intact cag pathogenicity island (PAI). The T68 strain, isolated from a Japanese patient at our institution, was negative for the above two virulence factors and lacked cag PAI. An isogenic cagE negative mutant (TN2-ΔcagE) was constructed by inserting a kanamycin resistant gene cassette into the cagE locus of cag PAI of TN2. The isogenic vacA negative mutant (TN2-ΔvacA) was constructed by disrupting the vacA gene of TN2. Luciferase activity is presented as a fold induction relative to basal levels measured in untreated cells. Mean (SD) values of four independent experiments are shown. *p<0.05 compared with TN2 by Dunnett's multiple comparison.
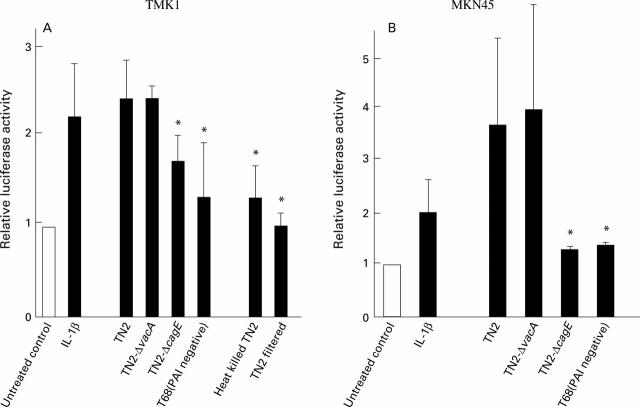
Figure 2 .
Transactivation of AP-1 induced by Helicobacter pylori. Transactivation of AP-1 in TMK1 (A) and MKN45 (B) cells induced by H pylori was measured by luciferase assay using pAP-1-Luc. The TN2 strain was positive for two known virulence factors, CagA and VacA, and had intact cag pathogenicity island (PAI). The T68 strain, isolated from a Japanese patient at our institution, was negative for the above two virulence factors and lacked cag PAI. An isogenic cagE negative mutant (TN2-ΔcagE) was constructed by inserting a kanamycin resistant gene cassette into the cagE locus of cag PAI of TN2. The isogenic vacA negative mutant (TN2-ΔvacA) was constructed by disrupting the vacA gene of TN2. Luciferase activity is presented as a fold induction relative to basal levels measured in untreated cells. Mean (SD) values of four independent experiments are shown. *p<0.05 compared with TN2 by Dunnett's multiple comparison.
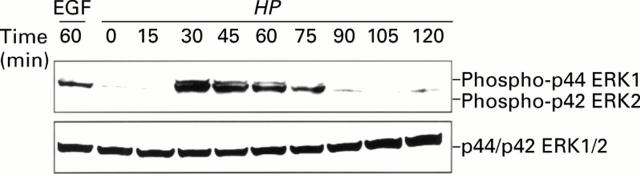
Figure 3 .
Phosphorylation of ERK1/2 by Helicobacter pylori (HP). Immunoblot analysis showing phospho-ERK1/2 (top panel) and total ERK1/2 (bottom panel) in TMK1 cells cocultured with H pylori. Antibodies used react with both ERK1 and ERK2. Stimulation by epidermal growth factor (EGF 10 ng/ml for 60 minutes) was used as a positive control.
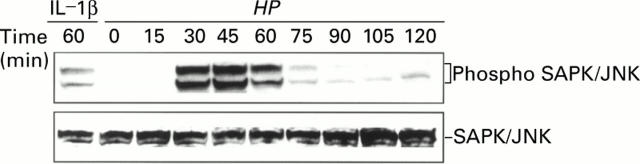
Figure 4 .
Phosphorylation of JNK/SAPK by Helicobacter pylori (HP). Immunoblot analysis showing phospho JNK (top panel) and total JNK (bottom panel) in TMK1 cells cocultured with H pylori. Stimulation by interleukin 1β (IL-1β 10 ng/ml for 60 minutes) was used as a positive control.
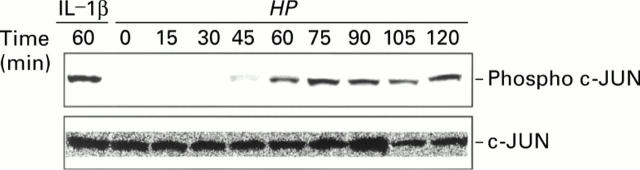
Figure 5 .
Phosphorylation of c-Jun by Helicobacter pylori (HP). Immunoblot analysis showing phospho c-JUN (top panel) and total c-JUN (bottom panel) in TMK1 cells cocultured with H pylori. Stimulation by interleukin 1β (IL-1β 10 ng/ml for 60 min) was used as a positive control.
Figure 6 .
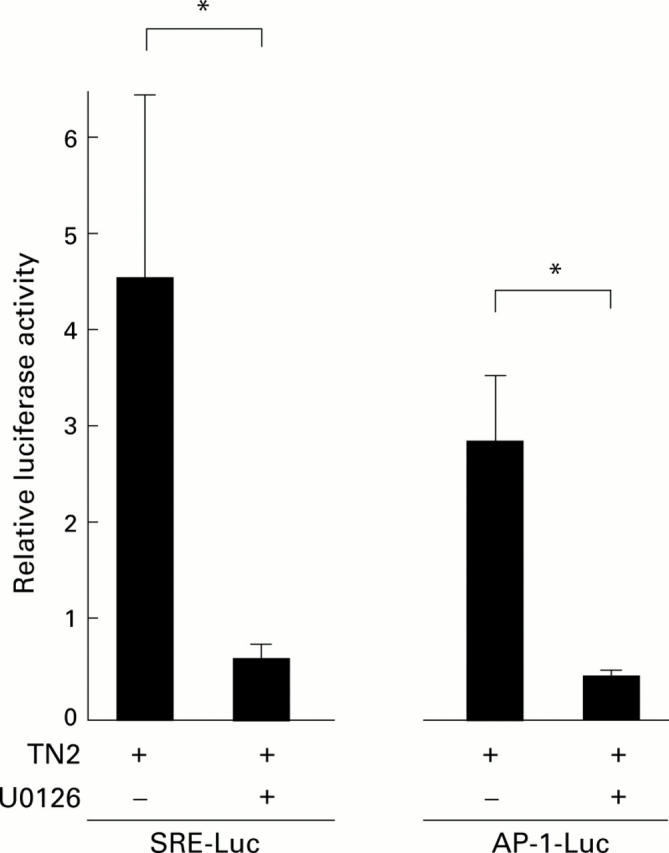
Effects of MEK inhibitor on Helicobacter pylori induced transactivation of SRE and AP-1. Transactivation of SRE and AP-1 in TMK1 cells induced by H pylori was measured by luciferase assay using pSRE-Luc and pAP-1-Luc with (+) or without (−) the MEK inhibitor U0126 (10 µM). Luciferase activity is presented as a fold induction relative to basal levels measured in untreated cells. Mean (SD) values of three independent experiments are shown. *p<0.05 by unpaired Student's t test.
Figure 7 .
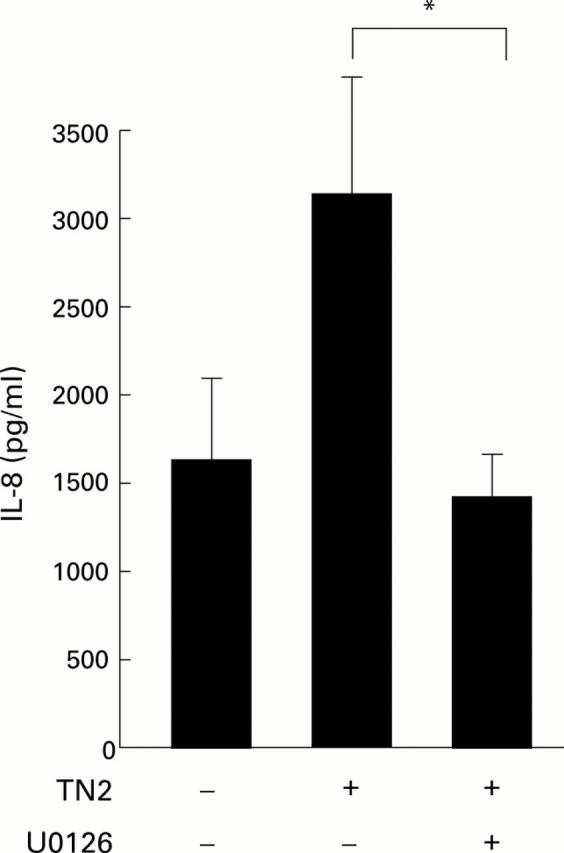
Effects of MEK inhibitor on Helicobacter pylori induced interleukin 8 (IL-8) secretion. TMK1 cells were cultured alone as control or were cocultured with H pylori with (+) or without (−) the MEK inhibitor U0126 (10 µM). Supernatant IL-8 concentrations are shown. Mean (SD) values of three independent experiments. *p<0.05 by unpaired Student's t test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aihara M., Tsuchimoto D., Takizawa H., Azuma A., Wakebe H., Ohmoto Y., Imagawa K., Kikuchi M., Mukaida N., Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect Immun. 1997 Aug;65(8):3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D., Kharbanda S., Uchiyama H., Urashima M., Fragoso R., Sen J., Kufe D. W., Anderson K. C. Identification of upstream signals regulating interleukin-6 gene expression during in vitro treatment of human B cells with pokeweed mitogen. Blood. 1994 Oct 1;84(7):2243–2252. [PubMed] [Google Scholar]
- Covacci A., Telford J. L., Del Giudice G., Parsonnet J., Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999 May 21;284(5418):1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Farmery S. M., Lindley I. J., Figura N., Peichl P., Tompkins D. S. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994 Oct;47(10):945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Heatley R. V., Wyatt J. I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991 Dec;32(12):1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata M. F., Horiuchi K. Y., Manos E. J., Daulerio A. J., Stradley D. A., Feeser W. S., Van Dyk D. E., Pitts W. J., Earl R. A., Hobbs F. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998 Jul 17;273(29):18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Vaira D., Campieri M., Holton J., Menegatti M., Belluzzi A., Bertinelli E., Ferretti M., Brignola C., Miglioli M. Enhanced mucosal interleukin-6 and -8 in Helicobacter pylori-positive dyspeptic patients. Am J Gastroenterol. 1994 Jun;89(6):883–887. [PubMed] [Google Scholar]
- Glocker E., Lange C., Covacci A., Bereswill S., Kist M., Pahl H. L. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-kappaB activation. Infect Immun. 1998 May;66(5):2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. S., Wynne J., Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995 Jun 30;81(7):1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Huang J., O'Toole P. W., Doig P., Trust T. J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995 May;63(5):1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992 Feb 15;148(4):1240–1250. [PubMed] [Google Scholar]
- Keates S., Hitti Y. S., Upton M., Kelly C. P. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997 Oct;113(4):1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- Liao J., Hodge C., Meyer D., Ho P. S., Rosenspire K., Schwartz J. Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element. J Biol Chem. 1997 Oct 10;272(41):25951–25958. doi: 10.1074/jbc.272.41.25951. [DOI] [PubMed] [Google Scholar]
- Maeda S., Ogura K., Yoshida H., Kanai F., Ikenoue T., Kato N., Shiratori Y., Omata M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998 Mar;42(3):338–343. doi: 10.1136/gut.42.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Yoshida H., Ikenoue T., Ogura K., Kanai F., Kato N., Shiratori Y., Omata M. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut. 1999 Mar;44(3):336–341. doi: 10.1136/gut.44.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Meyer-ter-Vehn T., Covacci A., Kist M., Pahl H. L. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J Biol Chem. 2000 May 26;275(21):16064–16072. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Legon S., Davies J., Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994 Nov;35(11):1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann M., Wessler S., Bartsch C., Wieland B., Covacci A., Haas R., Meyer T. F. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J Biol Chem. 1999 Oct 29;274(44):31655–31662. doi: 10.1074/jbc.274.44.31655. [DOI] [PubMed] [Google Scholar]
- Ochiai A., Yasui W., Tahara E. Growth-promoting effect of gastrin on human gastric carcinoma cell line TMK-1. Jpn J Cancer Res. 1985 Nov;76(11):1064–1071. [PubMed] [Google Scholar]
- Ogura K., Takahashi M., Maeda S., Ikenoue T., Kanai F., Yoshida H., Shiratori Y., Mori K., Mafune K. I., Omata M. Interleukin-8 production in primary cultures of human gastric epithelial cells induced by Helicobacter pylori. Dig Dis Sci. 1998 Dec;43(12):2738–2743. doi: 10.1023/a:1026671815512. [DOI] [PubMed] [Google Scholar]
- Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994 Jun;15(6):274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Segal E. D., Cha J., Lo J., Falkow S., Tompkins L. S. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A. 1999 Dec 7;96(25):14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y., Torti S. V., Torti F. M. Activation of the ferritin H enhancer, FER-1, by the cooperative action of members of the AP1 and Sp1 transcription factor families. J Biol Chem. 1998 Jan 30;273(5):2984–2992. doi: 10.1074/jbc.273.5.2984. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Watanabe T., Tada M., Nagai H., Sasaki S., Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998 Sep;115(3):642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]