Abstract
BACKGROUND—The development of clinically significant disease in South Africa is associated with the vacuolating cytotoxin gene (vacA) s1 genotype but not with the presence of the cytotoxin associated gene cagA. cagA occurs in >95% of South African isolates and is a variable marker for the entire cag pathogenicity island (PAI). AIM—To characterise the cagPAI in South African isolates and to investigate if structural variants of this multigene locus were associated with variations in vacA status and clinical outcome. PATIENTS AND METHODS—We studied 109 Helicobacter pylori strains (36 from patients with peptic ulceration, 26 with gastric adenocarcinoma, and 47 with no pathology other than gastritis) for differences in selected genes of the cagPAI and alleles of vacA by polymerase chain reaction. RESULTS—All strains were cagA+. Sixty five (60%) strains had an intact contiguous cagPAI; 78% of peptic ulcer isolates, 73% of gastric adenocarcinoma isolates, but only 40% of gastritis alone isolates (p< 0.01). The entire cagII region was undetectable in 23% of gastritis alone isolates but in only 8% of peptic ulceration isolates (p<0.05). The vacA signal sequence and mid region demonstrated a strong relationship between the virulence associated vacA s1 (p<0.005) and vacA m1 (p=0.05) alleles and an intact cagPAI. CONCLUSION—Although a complete cagPAI was a feature of most infected individuals, deletions in the 5' region of this genetic locus were associated with gastritis alone and with the non-cytotoxic s2/m2 vacA genotype. Keywords: cagA; gastric cancer; Helicobacter pylori; pathogenicity; peptic ulcer; vacuolating cytotoxin
Full Text
The Full Text of this article is available as a PDF (156.3 KB).
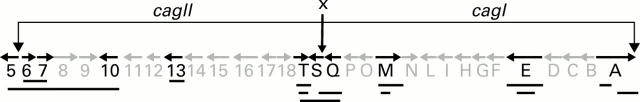
Figure 1 .
Schematic diagram of the cag pathogenicity island with the putative junction site between cagQ~S where IS605 may be located (vertical arrow, x) noted in NCTC11638. Genes and positions are from GenBank accession number AC000108 (cagII) and U60176 (cagI). Polymerase chain reaction amplimers analysed in this study are denoted by bold horizontal lines beneath the genes.
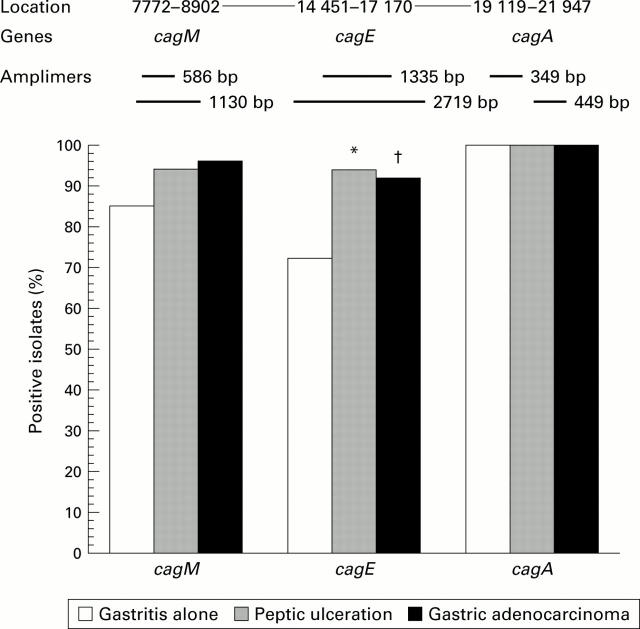
Figure 2 .
Schematic diagram of cagI with the polymerase chain reaction amplimers of the three genes analysed (top). Genes and positions are from GenBank accession number U60176. Prevalence of target genes in the different disease groups is shown below. *p<0.009, †p<0.05 versus gastritis alone; Fisher's test.
Figure 3 .
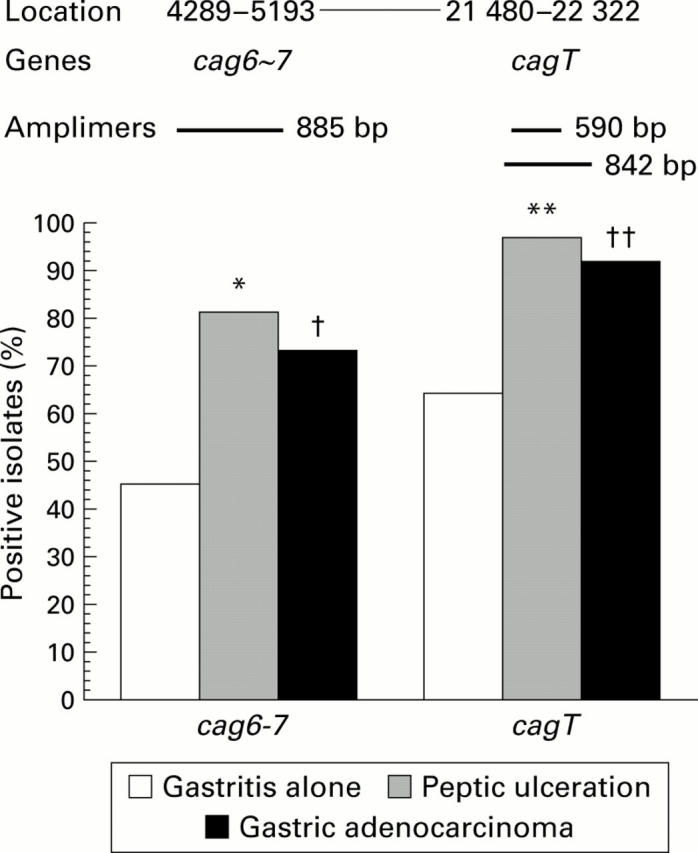
Schematic diagram of cagII with the polymerase chain reaction amplimers of the three genes analysed (top). Genes and positions are from GenBank accession number AC000108. Prevalence of target genes in the different disease groups is shown below. *p<0.0002, **p<0.0009, †p<0.007, ††p<0.05 versus gastritis alone; Fisher's test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Azuma T., Berg D. E., Ito Y., Morelli G., Pan Z. J., Suerbaum S., Thompson S. A., van der Ende A., van Doorn L. J. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol Microbiol. 1999 May;32(3):459–470. doi: 10.1046/j.1365-2958.1999.01382.x. [DOI] [PubMed] [Google Scholar]
- Akopyants N. S., Clifton S. W., Kersulyte D., Crabtree J. E., Youree B. E., Reece C. A., Bukanov N. O., Drazek E. S., Roe B. A., Berg D. E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998 Apr;28(1):37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- Alm R. A., Ling L. S., Moir D. T., King B. L., Brown E. D., Doig P. C., Smith D. R., Noonan B., Guild B. C., deJonge B. L. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999 Jan 14;397(6715):176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- Asahi M., Azuma T., Ito S., Ito Y., Suto H., Nagai Y., Tsubokawa M., Tohyama Y., Maeda S., Omata M. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000 Feb 21;191(4):593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J. C. The clinical relevance of strain types of Helicobacter pylori. Gut. 1997 Jun;40(6):701–703. doi: 10.1136/gut.40.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995 May 15;55(10):2111–2115. [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J Exp Med. 2000 Feb 21;191(4):587–592. doi: 10.1084/jem.191.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- El-Mahdi A. M., Patchett S. E., Char S., Domizio P., Fedail S. S., Kumar P. J. Does CagA contribute to ulcer pathogenesis in a developing country, such as Sudan? Eur J Gastroenterol Hepatol. 1998 Apr;10(4):313–316. doi: 10.1097/00042737-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Jenks P. J., Mégraud F., Labigne A. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut. 1998 Dec;43(6):752–758. doi: 10.1136/gut.43.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M., Lastovica A. J., Atherton J. C., Louw J. A. Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut. 1999 Oct;45(4):499–502. doi: 10.1136/gut.45.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Letley D. P., Lastovica A., Louw J. A., Hawkey C. J., Atherton J. C. Allelic diversity of the Helicobacter pylori vacuolating cytotoxin gene in South Africa: rarity of the vacA s1a genotype and natural occurrence of an s2/m1 allele. J Clin Microbiol. 1999 Apr;37(4):1203–1205. doi: 10.1128/jcm.37.4.1203-1205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Yoshida H., Ikenoue T., Ogura K., Kanai F., Kato N., Shiratori Y., Omata M. Structure of cag pathogenicity island in Japanese Helicobacter pylori isolates. Gut. 1999 Mar;44(3):336–341. doi: 10.1136/gut.44.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenbreit S., Püls J., Sedlmaier B., Gerland E., Fischer W., Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000 Feb 25;287(5457):1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Rudi J., Kolb C., Maiwald M., Kuck D., Sieg A., Galle P. R., Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998 Apr;36(4):944–948. doi: 10.1128/jcm.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E. D., Cha J., Lo J., Falkow S., Tompkins L. S. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A. 1999 Dec 7;96(25):14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E., Owen R. J., Williams M., Pounder R. E. Conservation of the cag pathogenicity island of Helicobacter pylori: associations with vacuolating cytotoxin allele and IS605 diversity. Gastroenterology. 1999 Dec;117(6):1308–1315. doi: 10.1016/s0016-5085(99)70281-7. [DOI] [PubMed] [Google Scholar]
- Stein M., Rappuoli R., Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A. 2000 Feb 1;97(3):1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomb J. F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleischmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997 Aug 7;388(6642):539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Warnich L., Kotze M. J., Groenewald I. M., Groenewald J. Z., van Brakel M. G., van Heerden C. J., de Villiers J. N., van de Ven W. J., Schoenmakers E. F., Taketani S. Identification of three mutations and associated haplotypes in the protoporphyrinogen oxidase gene in South African families with variegate porphyria. Hum Mol Genet. 1996 Jul;5(7):981–984. doi: 10.1093/hmg/5.7.981. [DOI] [PubMed] [Google Scholar]
- Weel J. F., van der Hulst R. W., Gerrits Y., Roorda P., Feller M., Dankert J., Tytgat G. N., van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996 May;173(5):1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- Wirth H. P., Yang M., Karita M., Blaser M. J. Expression of the human cell surface glycoconjugates Lewis x and Lewis y by Helicobacter pylori isolates is related to cagA status. Infect Immun. 1996 Nov;64(11):4598–4605. doi: 10.1128/iai.64.11.4598-4605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon A. C., Ortiz-Hidalgo C., Falzon M. R., Isaacson P. G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991 Nov 9;338(8776):1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Osato M. S., Sepulveda A. R., Gutierrez O., Figura N., Kim J. G., Kodama T., Kashima K., Graham D. Y. Molecular epidemiology of Helicobacter pylori: separation of H. pylori from East Asian and non-Asian countries. Epidemiol Infect. 2000 Feb;124(1):91–96. doi: 10.1017/s0950268899003209. [DOI] [PMC free article] [PubMed] [Google Scholar]