Abstract
Tryptic peptides of the novel ceftazidimase CAZ-5 were sequenced by manual Edman degradation and aligned according to strong homology (more than 98%) with SHV-1 and SHV-2 beta-lactamase sequences. CAZ-5 differed from SHV-1 by five amino acid substitutions. Unusually high activity of CAZ-5 towards ceftazidime was imputed to substitution of a Lys for a Glu at position 214 of the mature protein.
Full text
PDF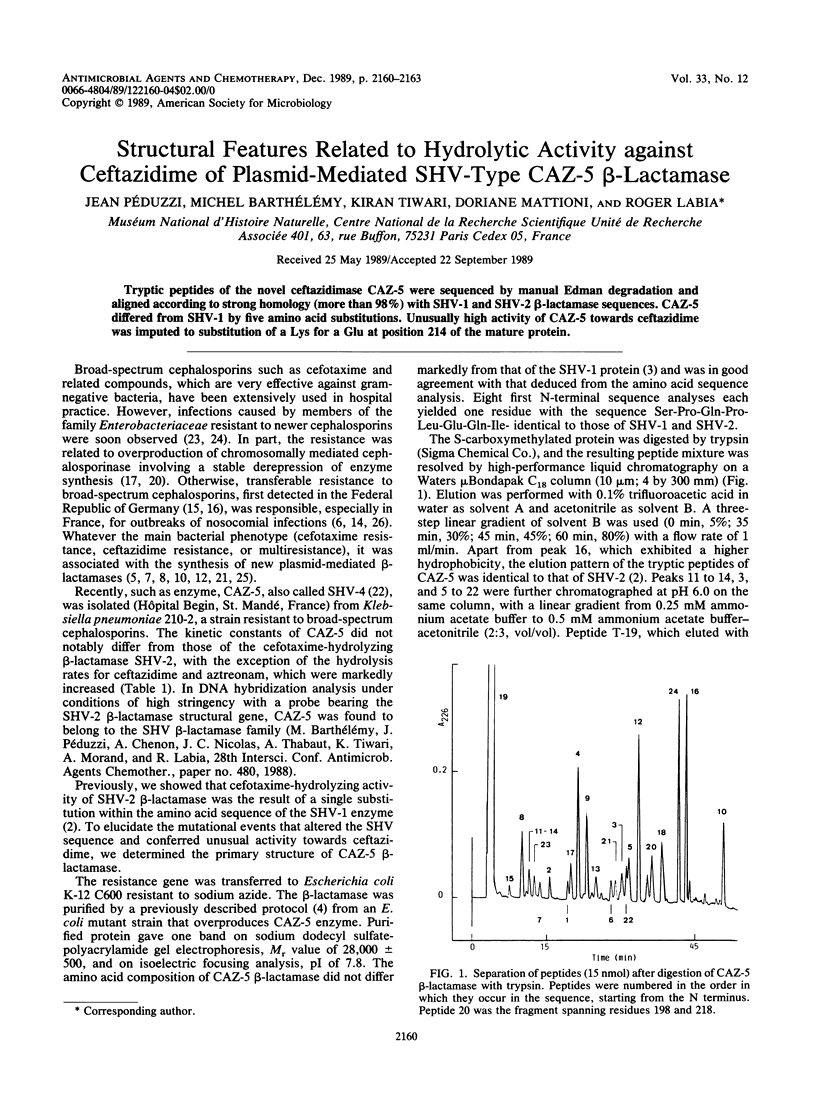
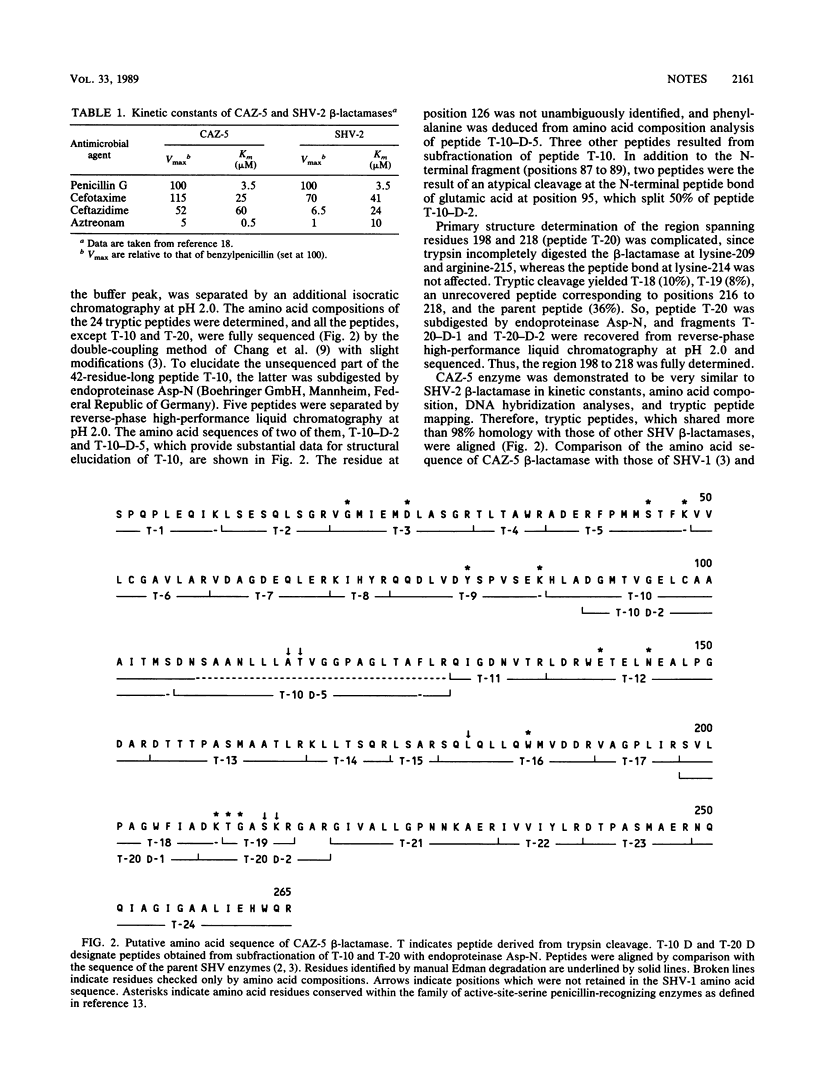
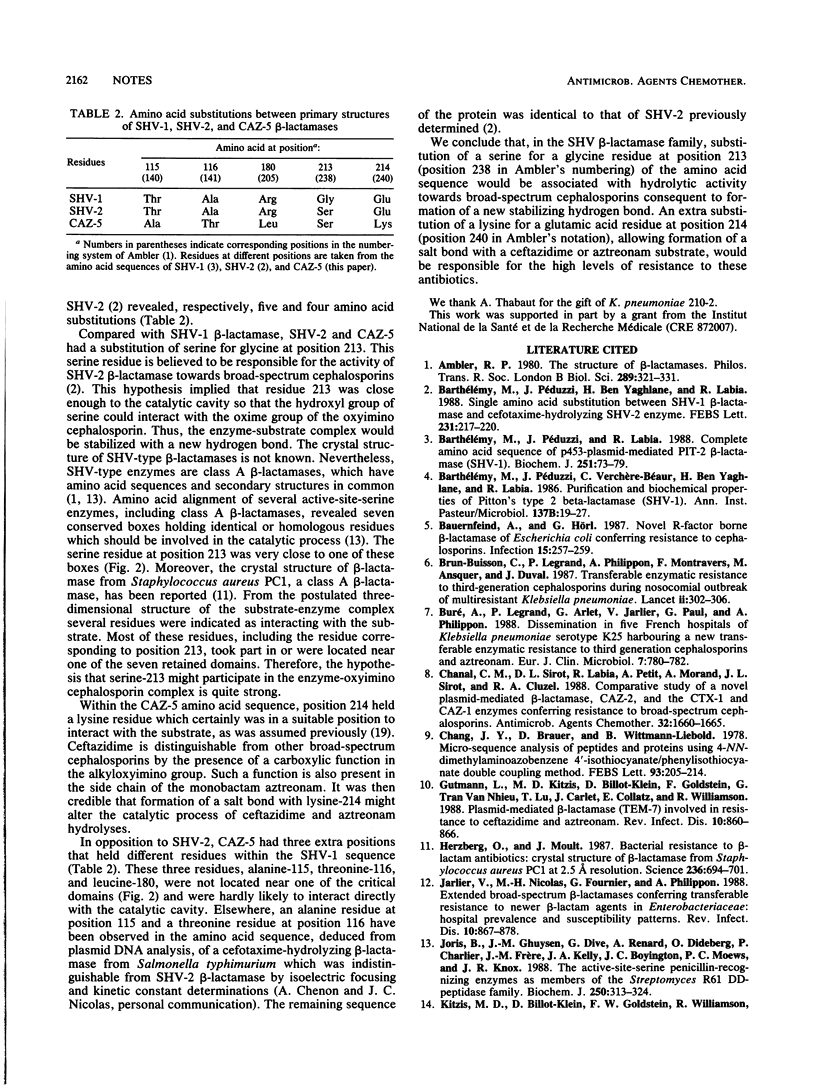

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Barthélémy M., Peduzzi J., Labia R. Complete amino acid sequence of p453-plasmid-mediated PIT-2 beta-lactamase (SHV-1). Biochem J. 1988 Apr 1;251(1):73–79. doi: 10.1042/bj2510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy M., Peduzzi J., Verchère-Beaur C., Ben Yaghlane H., Labia R. Purification and biochemical properties of Pitton's type 2 beta-lactamase (SHV-1). Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):19–27. doi: 10.1016/s0769-2609(86)80090-4. [DOI] [PubMed] [Google Scholar]
- Barthélémy M., Péduzzi J., Ben Yaghlane H., Labia R. Single amino acid substitution between SHV-1 beta-lactamase and cefotaxime-hydrolyzing SHV-2 enzyme. FEBS Lett. 1988 Apr 11;231(1):217–220. doi: 10.1016/0014-5793(88)80734-8. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Hörl G. Novel R-factor borne beta-lactamase of Escherichia coli confering resistance to cephalosporins. Infection. 1987 Jul-Aug;15(4):257–259. doi: 10.1007/BF01644127. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C., Legrand P., Philippon A., Montravers F., Ansquer M., Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987 Aug 8;2(8554):302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- Buré A., Legrand P., Arlet G., Jarlier V., Paul G., Philippon A. Dissemination in five French hospitals of Klebsiella pneumoniae serotype K25 harbouring a new transferable enzymatic resistance to third generation cephalosporins and aztreonam. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):780–782. doi: 10.1007/BF01975048. [DOI] [PubMed] [Google Scholar]
- Chanal C. M., Sirot D. L., Labia R., Petit A., Morand A., Sirot J. L., Cluzel R. A. Comparative study of a novel plasmid-mediated beta-lactamase, CAZ-2, and the CTX-1 and CAZ-1 enzymes conferring resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1988 Nov;32(11):1660–1665. doi: 10.1128/aac.32.11.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Kitzis M. D., Billot-Klein D., Goldstein F., Tran Van Nhieu G., Lu T., Carlet J., Collatz E., Williamson R. Plasmid-mediated beta-lactamase (TEM-7) involved in resistance to ceftazidime and aztreonam. Rev Infect Dis. 1988 Jul-Aug;10(4):860–866. doi: 10.1093/clinids/10.4.860. [DOI] [PubMed] [Google Scholar]
- Herzberg O., Moult J. Bacterial resistance to beta-lactam antibiotics: crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.5 A resolution. Science. 1987 May 8;236(4802):694–701. doi: 10.1126/science.3107125. [DOI] [PubMed] [Google Scholar]
- Jarlier V., Nicolas M. H., Fournier G., Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988 Jul-Aug;10(4):867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- Joris B., Ghuysen J. M., Dive G., Renard A., Dideberg O., Charlier P., Frère J. M., Kelly J. A., Boyington J. C., Moews P. C. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988 Mar 1;250(2):313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzis M. D., Billot-Klein D., Goldstein F. W., Williamson R., Tran Van Nhieu G., Carlet J., Acar J. F., Gutmann L. Dissemination of the novel plasmid-mediated beta-lactamase CTX-1, which confers resistance to broad-spectrum cephalosporins, and its inhibition by beta-lactamase inhibitors. Antimicrob Agents Chemother. 1988 Jan;32(1):9–14. doi: 10.1128/aac.32.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebe C., Nies B. A., Meyer J. F., Tolxdorff-Neutzling R. M., Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe H., Shah P., Krcmery V., Antal M., Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983 Nov-Dec;11(6):315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- Korfmann G., Kliebe C., Wiedemann B. Beta-lactam antibiotics and selection of resistance: speculation on the evolution of R-plasmids. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):113–121. doi: 10.1093/jac/18.supplement_c.113. [DOI] [PubMed] [Google Scholar]
- Labia R., Barthélémy M., Peduzzi J., Morand A., Tiwari K. Comportement de la ceftazidime à l'égard de diverses classes de bêtalactamases. Situation en 1988. Presse Med. 1988 Oct 26;17(37):1890–1894. [PubMed] [Google Scholar]
- Labia R., Morand A., Tiwari K., Sirot J., Sirot D., Petit A. Interactions of new plasmid-mediated beta-lactamases with third-generation cephalosporins. Rev Infect Dis. 1988 Jul-Aug;10(4):885–891. doi: 10.1093/clinids/10.4.885. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur J Clin Microbiol. 1987 Aug;6(4):439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- Petit A., Sirot D. L., Chanal C. M., Sirot J. L., Labia R., Gerbaud G., Cluzel R. A. Novel plasmid-mediated beta-lactamase in clinical isolates of Klebsiella pneumoniae more resistant to ceftazidime than to other broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1988 May;32(5):626–630. doi: 10.1128/aac.32.5.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A., Labia R., Jacoby G. Extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1989 Aug;33(8):1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Sirot J., Chanal C., Petit A., Sirot D., Labia R., Gerbaud G. Klebsiella pneumoniae and other Enterobacteriaceae producing novel plasmid-mediated beta-lactamases markedly active against third-generation cephalosporins: epidemiologic studies. Rev Infect Dis. 1988 Jul-Aug;10(4):850–859. doi: 10.1093/clinids/10.4.850. [DOI] [PubMed] [Google Scholar]


