Abstract
BACKGROUND—Activation of hepatic stellate cells (HSCs) to a myofibroblastic phenotype is a key event in liver fibrosis. Identification of transcription factors with activities that are modulated during HSC activation will improve our understanding of the molecular events controlling HSC activation. AIMS—To determine if changes in E-box DNA binding activity occur during in vitro and in vivo activation of rat and human HSCs and to investigate mechanisms underlying any observed changes. METHODS—Nuclear extracts were prepared from rat HSCs isolated and cultured from normal and carbon tetrachloride injured rat livers and from HSCs isolated from human liver. EMSA analysis of E-box DNA binding activity was performed on nuclear extracts to determine changes during HSC activation. Western and northern blot analysis of MyoD and Id1 basic helix-loop-helix (bHLH) proteins was performed to confirm expression in HSC. RESULTS—HSC activation was associated with inducible expression of two low mobility E-box binding complexes that were immunoreactive with an anti-MyoD antibody. MyoD mRNA expression was found at similar levels in freshly isolated and activated HSCs; in contrast, MyoD protein expression was elevated in activated HSCs. Activation of rat HSCs was accompanied by reduced expression of the inhibitory bHLH protein Id1. CONCLUSIONS—In vitro and in vivo activation of rat and human HSCs is accompanied by induction of MyoD binding to E-box DNA sequences which appears to be mechanistically associated with elevated MyoD protein expression and reduced expression of the inhibitory Id1 protein. Clarification of the role of MyoD and Id1 proteins in HSC activation and liver fibrogenesis is now required. Keywords: liver fibrosis; hepatic stellate cell; basic helix-loop-helix transcription factors; MyoD; Id1
Full Text
The Full Text of this article is available as a PDF (191.9 KB).
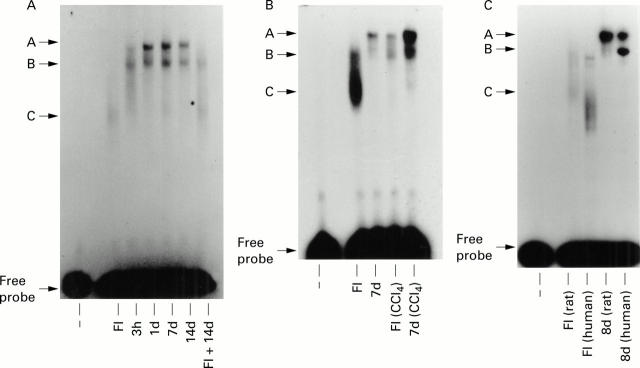
Figure 1 .
Analysis of E-box DNA binding activities in primary rat and human hepatic stellate cells (HSCs). (A) Modulation of E-box DNA binding in cultured rat HSCs. Nuclear extracts (10 µg) prepared from freshly isolated (FI) and cultured (three hours to 14 days) rat HSCs were used to detect E-box DNA binding activities by EMSA. A control track (−) lacking nuclear extract was included on the far left hand side of the gel. FI+14 days shows the effects of adding equal quantities (10 µg of each) of nuclear extract into the same EMSA reaction mixture. A, B, and C denote retarded E-box DNA:protein binding complexes. (B) Modulation of E-box DNA binding during in vivo activation of rat HSCs. Control and carbon tetrachloride (CCl4) treated rats (48 hours) were used to prepare HSCs which were either harvested (FI and FI (CCl4)) or cultured for seven days (7d and 7d (CCl4)) prior to preparation of nuclear extracts; 10 µg of each extract were used for electrophoretic mobility shift assay (EMSA). (C) Comparison of rat and human HSC E-box DNA binding activities: 10 µg of nuclear extract prepared from freshly isolated (FI) or eight day cultured (8d) rat and human HSCs were used in EMSA assays. All gels are representative of at least three independent experiments.
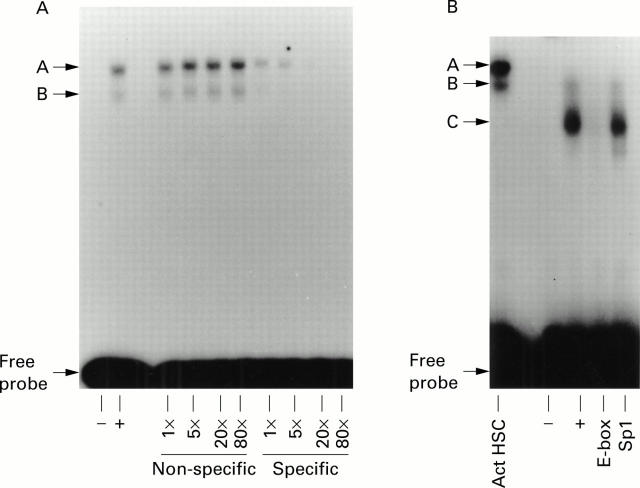
Figure 2 .
Determination of the specificity of hepatic stellate cell (HSC) E-box DNA:protein complexes. (A) Competition electrophoretic mobility shift assay (EMSA) was performed using nuclear extracts from activated rat HSCs and up to an 80-fold excess of specific (E-box) or non-specific (Sp1) unlabelled double stranded oligonucleotides as competitors. (B) Competition EMSA performed with nuclear extracts from freshly isolated rat HSCs using an 80-fold excess of specific (E-box) or non-specific (Sp1) oligonucleotides. Both gels are representative of at least three independent experiments.
Figure 3 .
Sequence requirements for mE-box DNA binding. (A) Competition assay between mE-box and α-smooth muscle actin (α-SMA) E-box. Nuclear extract from activated rat hepatic stellate cells (HSCs) was initially incubated in the presence of an 80-fold excess of unlabelled wild type mE-box, mutant (Δ) mE-box, α-SMA E-box, or mutant (Δ) α-SMA E-box prior to incubation with radiolabelled wild type mE-box probe. (B) Competition assay between mE-box and mα-SMA E-box. Nuclear extract from activated rat HSCs was initially incubated with an 80-fold excess of unlabelled wild type mE-box or chimeric mα-SMA E-box oligonucleotides prior to incubation with radiolabelled mE-box probe. Gels are representative of two independent experiments.
Figure 4 .
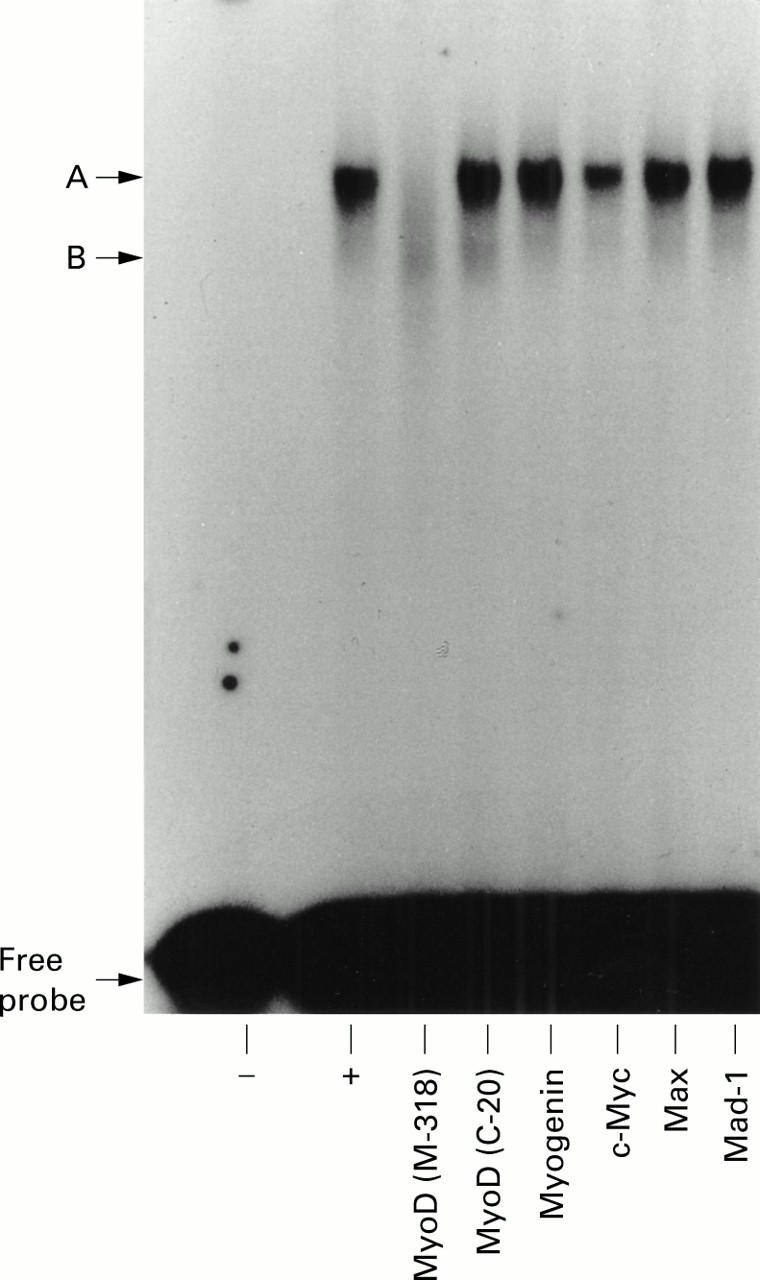
Reactivity of hepatic stellate cell (HSC) mE-box complexes with an anti-MyoD antibody. Electrophoretic mobility shift assay (EMSA) reactions consisting of nuclear extract from activated rat HSCs and wild type mE-box probe were incubated for 16 hours at 4°C either unsupplemented (+) or supplemented with polyclonal antibodies recognising MyoD (M318 or C-20), myogenin, c-Myc, Max, and Mad-1. The gel is representative of two independent experiments.
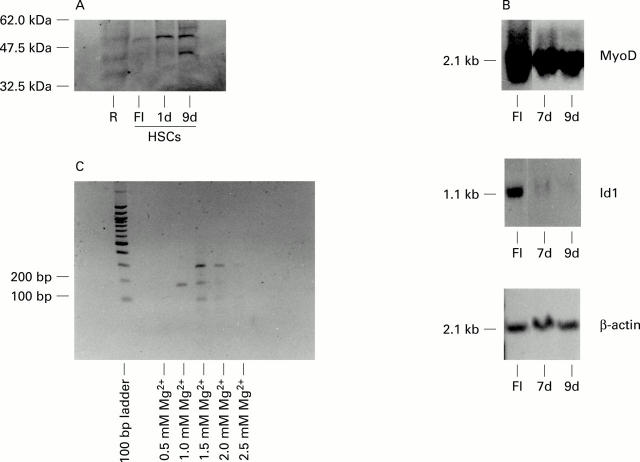
Figure 5 .
Regulation of MyoD expression in rat hepatic stellate cells (HSCs). (A) Immunoblot detection of MyoD protein expression in rat HSCs. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis and immunoblotting were performed as described in methods using equal quantities of nuclear protein extracts from RAW264.7 macrophages (R), freshly isolated rat HSCs (FI), and rat HSC cultured for one (1d) or nine (9d) days on plastic. (B) Northern blot detection of MyoD mRNA in rat HSCs. Northern blotting was performed as described in methods using equal quantities of whole cell RNA extracted from freshly isolated (FI) rat HSCs and rat HSCs cultured on plastic for either seven (7d) or nine (9d) days. Filters were probed with radiolabelled cDNA probes for MyoD, Id1, and β-actin. (C) Reverse transcription-polymerase chain reaction (RT-PCR) detection of MyoD mRNA in activated rat HSCs. Following reverse transcription, equal quantities of cDNA were amplified using primers spanning nucleotides 468 to 642 of the MyoD cDNA sequence and in the presence of 0.5 to 2.5 mM MgCl2. The single 174 bp PCR product obtained using 1.0 mM MgCl2 was subcloned into pcDNA3 and confirmed as exon 1 derived MyoD sequence by DNA sequence analysis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu Hatoum O., Gross-Mesilaty S., Breitschopf K., Hoffman A., Gonen H., Ciechanover A., Bengal E. Degradation of myogenic transcription factor MyoD by the ubiquitin pathway in vivo and in vitro: regulation by specific DNA binding. Mol Cell Biol. 1998 Oct;18(10):5670–5677. doi: 10.1128/mcb.18.10.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr M. J., Vincent K. J., Arthur M. J., Fowler A. V., Smart D. E., Wright M. C., Clark I. M., Benyon R. C., Iredale J. P., Mann D. A. Control of the tissue inhibitor of metalloproteinases-1 promoter in culture-activated rat hepatic stellate cells: regulation by activator protein-1 DNA binding proteins. Hepatology. 1999 Mar;29(3):839–848. doi: 10.1002/hep.510290333. [DOI] [PubMed] [Google Scholar]
- Bataller R., Nicolás J. M., Ginès P., Esteve A., Nieves Görbig M., Garcia-Ramallo E., Pinzani M., Ros J., Jiménez W., Thomas A. P. Arginine vasopressin induces contraction and stimulates growth of cultured human hepatic stellate cells. Gastroenterology. 1997 Aug;113(2):615–624. doi: 10.1053/gast.1997.v113.pm9247484. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Biggs T. E., Cooke S. J., Barton C. H., Harris M. P., Saksela K., Mann D. A. Induction of activator protein 1 (AP-1) in macrophages by human immunodeficiency virus type-1 NEF is a cell-type-specific response that requires both hck and MAPK signaling events. J Mol Biol. 1999 Jul 2;290(1):21–35. doi: 10.1006/jmbi.1999.2849. [DOI] [PubMed] [Google Scholar]
- Bissell D. M. Hepatic fibrosis as wound repair: a progress report. J Gastroenterol. 1998 Apr;33(2):295–302. doi: 10.1007/s005350050087. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Bounpheng M. A., Dimas J. J., Dodds S. G., Christy B. A. Degradation of Id proteins by the ubiquitin-proteasome pathway. FASEB J. 1999 Dec;13(15):2257–2264. [PubMed] [Google Scholar]
- Boyd K. E., Wells J., Gutman J., Bartley S. M., Farnham P. J. c-Myc target gene specificity is determined by a post-DNAbinding mechanism. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13887–13892. doi: 10.1073/pnas.95.23.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Elsharkawy A. M., Wright M. C., Hay R. T., Arthur M. J., Hughes T., Bahr M. J., Degitz K., Mann D. A. Persistent activation of nuclear factor-kappaB in cultured rat hepatic stellate cells involves the induction of potentially novel Rel-like factors and prolonged changes in the expression of IkappaB family proteins. Hepatology. 1999 Sep;30(3):761–769. doi: 10.1002/hep.510300327. [DOI] [PubMed] [Google Scholar]
- Friedman S. L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000 Jan 28;275(4):2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- Friedman S. L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993 Jun 24;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Halazonetis T. D., Kandil A. N. Determination of the c-MYC DNA-binding site. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6162–6166. doi: 10.1073/pnas.88.14.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. Hypermethylated myoblasts specifically deficient in MyoD autoactivation as a consequence of instability of MyoD. Exp Cell Res. 1996 Jul 10;226(1):170–182. doi: 10.1006/excr.1996.0216. [DOI] [PubMed] [Google Scholar]
- Iredale J. P., Benyon R. C., Pickering J., McCullen M., Northrop M., Pawley S., Hovell C., Arthur M. J. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998 Aug 1;102(3):538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale J. P., Murphy G., Hembry R. M., Friedman S. L., Arthur M. J. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J Clin Invest. 1992 Jul;90(1):282–287. doi: 10.1172/JCI115850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993 Dec 3;75(5):827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- Langlands K., Yin X., Anand G., Prochownik E. V. Differential interactions of Id proteins with basic-helix-loop-helix transcription factors. J Biol Chem. 1997 Aug 8;272(32):19785–19793. doi: 10.1074/jbc.272.32.19785. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Buck M., Houglum K., Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995 Nov;96(5):2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer D. C., Leinwand L. A. Sarcomeric gene expression and contractility in myofibroblasts. J Cell Biol. 1997 Dec 15;139(6):1477–1484. doi: 10.1083/jcb.139.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato Y., Hasumura Y., Takeuchi J. The role of fat-storing cells in Disse space fibrogenesis in alcoholic liver disease. Hepatology. 1983 Jul-Aug;3(4):559–566. doi: 10.1002/hep.1840030414. [DOI] [PubMed] [Google Scholar]
- Monteil A., Chemin J., Bourinet E., Mennessier G., Lory P., Nargeot J. Molecular and functional properties of the human alpha(1G) subunit that forms T-type calcium channels. J Biol Chem. 2000 Mar 3;275(9):6090–6100. doi: 10.1074/jbc.275.9.6090. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- O'Regan S., Traiffort E., Ruat M., Cha N., Compaore D., Meunier F. M. An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1835–1840. doi: 10.1073/pnas.030339697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M. Hepatic stellate (ITO) cells: expanding roles for a liver-specific pericyte. J Hepatol. 1995 Jun;22(6):700–706. doi: 10.1016/0168-8278(95)80227-4. [DOI] [PubMed] [Google Scholar]
- Roberts V. J., Steenbergen R., Murre C. Localization of E2A mRNA expression in developing and adult rat tissues. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7583–7587. doi: 10.1073/pnas.90.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Wang N. P., Marx J., McNutt M. A., Rutledge J. C., Gown A. M. Expression of myogenic regulatory proteins (myogenin and MyoD1) in small blue round cell tumors of childhood. Am J Pathol. 1995 Dec;147(6):1799–1810. [PMC free article] [PubMed] [Google Scholar]
- Weiner J. A., Chen A., Davis B. H. Platelet-derived growth factor is a principal inductive factormodulating mannose 6-phosphate/insulin-like growth factor-II receptorgene expression via a distal E-box in activated hepatic stellate cells. Biochem J. 2000 Jan 15;345(Pt 2):225–231. [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993 Dec 31;75(7):1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Whiteside S. T., Epinat J. C., Rice N. R., Israël A. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel NF-kappa B activity. EMBO J. 1997 Mar 17;16(6):1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi Y., Namihisa T., Kuroda H., Komatsu I., Miyazaki A., Watanabe S., Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984 Jul-Aug;4(4):709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]