Abstract
BACKGROUND—Octreotide inhibits gall bladder emptying and prolongs intestinal transit. This leads to increases in the proportion of deoxycholic acid in, and cholesterol saturation of, gall bladder bile, factors that contribute to the pathogenesis of octreotide induced gall stones. AIMS—To see if an intestinal prokinetic, cisapride, could overcome these adverse effects of octreotide and if so, be considered as a candidate prophylactic drug for preventing iatrogenic gall bladder stones. METHODS—A randomised, double blind, placebo controlled, crossover design was used to examine the effects of cisapride (10 mg four times daily) on gall bladder emptying, mouth to caecum and large bowel transit times, and the proportions of deoxycholic acid and other bile acids, in fasting serum from: (i) control subjects (n=6), (ii) acromegalic patients not treated with octreotide (n=6), (iii) acromegalics on long term octreotide (n=8), and (iv) patients with constipation (n=8). RESULTS—Cisapride had no prokinetic effect on the gall bladder. In fact, it significantly increased both fasting and postprandial gall bladder volumes. However, it shortened mouth to caecum (from 176 (13) to 113 (11) minutes; p<0.001) and large bowel (from 50 (3.0) to 31 (3.4) h; p<0.001) transit times. It also reduced the proportion of deoxycholic acid in serum from 26 (2.3) to 15 (1.8)% (p<0.001), with a reciprocal increase in the proportion of cholic acid from 40 (3.5) to 51 (3.8)% (p<0.01). There were significant linear relationships between large bowel transit time and the proportions of deoxycholic acid (r=0.81; p<0.001) and cholic acid (r=−0.53; p<0.001) in fasting serum. INTERPRETATION/SUMMARY—Cisapride failed to overcome the adverse effects of octreotide on gall bladder emptying but it countered octreotide induced prolongation of small and large bowel transit. Therefore, if changes in intestinal transit contribute to the development of octreotide induced gall bladder stones, enterokinetics such as cisapride may prevent their formation. Keywords: cisapride; deoxycholic acid; octreotide; acromegaly; gall bladder stones; large bowel transit time
Full Text
The Full Text of this article is available as a PDF (159.3 KB).
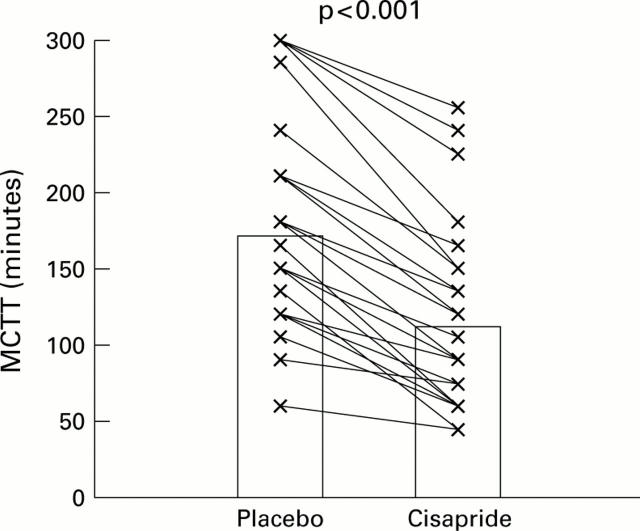
Figure 1 .
Paired data (individual data points with means) for mouth to caecum transit time (MCTT) in non-acromegalic control subjects (n=6), acromegalic patients not treated with octreotide (n=6), acromegalic patients on long term octreotide treatment (n=8), and patients with simple constipation (n=8), receiving placebo or cisapride (see text).
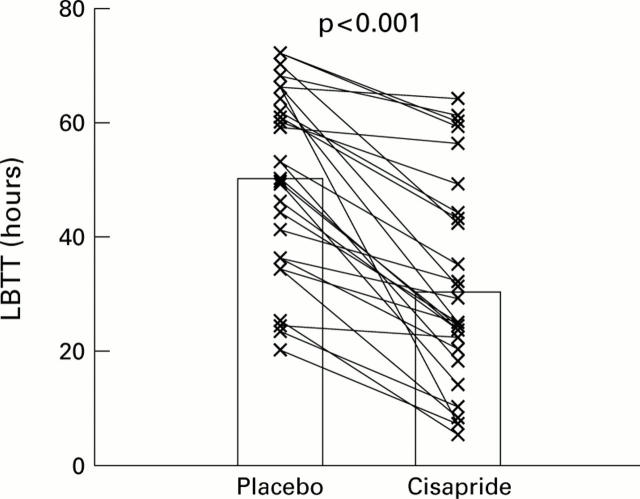
Figure 2 .
Paired data (individual data points with means) for large bowel transit time (LBTT) in non-acromegalic control subjects (n=6), acromegalic patients not treated with octreotide (n=6), acromegalic patients on long term octreotide treatment (n=8), and patients with simple constipation (n=8), receiving placebo or cisapride (see text).
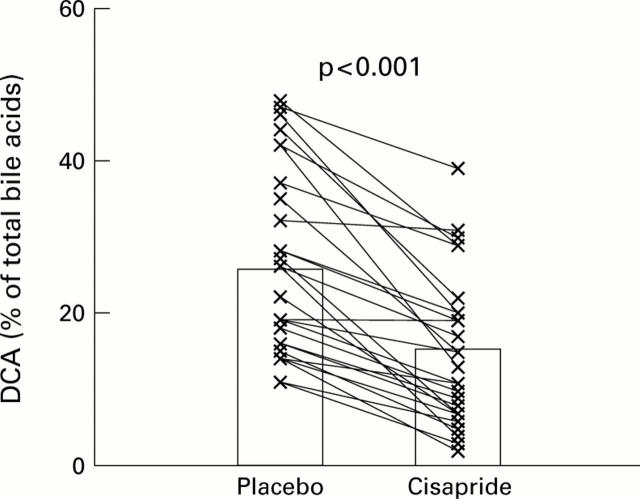
Figure 3 .
Paired data (individual data points with means) for the proportion of deoxycholic acid (DCA), expressed as a percentage of total serum bile acids in non-acromegalic control subjects (n=6), acromegalic patients not treated with octreotide (n=6), acromegalic patients on long term octreotide treatment (n=8), and patients with simple constipation (n=8), receiving placebo or cisapride (see text).
Figure 4 .
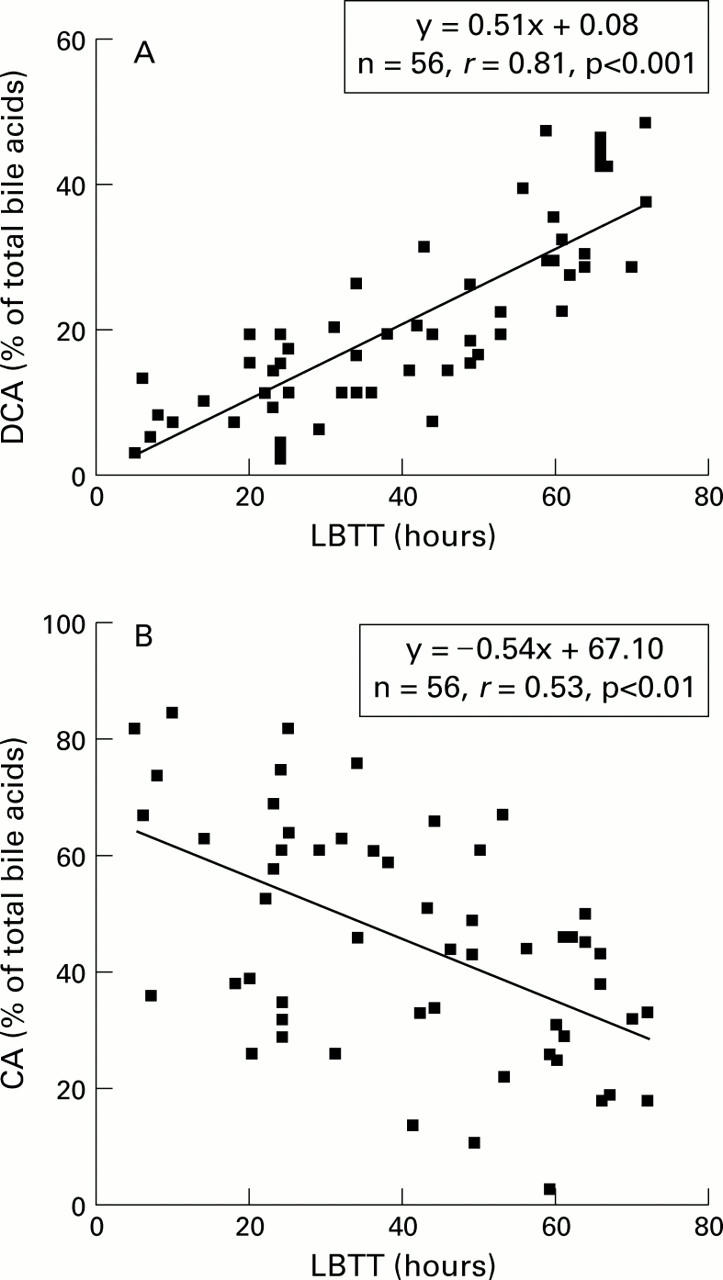
(A) Relationship between the proportion of deoxycholic acid (DCA) in fasting serum, expressed as a percentage of total bile acids, and large bowel transit time (LBTT). (B) Relationship between the proportion of cholic acid (CA) in fasting serum, expressed as a percentage of total bile acids, and LBTT.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzaroli F., Mazzella G., Mazzeo C., Simoni P., Festi D., Colecchia A., Montagnani M., Martino C., Villanova N., Roda A. Sluggish small bowel motility is involved in determining increased biliary deoxycholic acid in cholesterol gallstone patients. Am J Gastroenterol. 1999 Sep;94(9):2453–2459. doi: 10.1111/j.1572-0241.1999.01375.x. [DOI] [PubMed] [Google Scholar]
- Baeyens R., Reyntjens A., Verlinden M. Cisapride accelerates gastric emptying and mouth-to-caecum transit of a barium meal. Eur J Clin Pharmacol. 1984;27(3):315–318. doi: 10.1007/BF00542167. [DOI] [PubMed] [Google Scholar]
- Bazzoli F., Fromm H., Roda A., Tunuguntla A. K., Roda E., Barbara L., Amin P. Value of serum determinations for prediction of increased ursodeoxycholic and chenodeoxycholic levels in bile. Dig Dis Sci. 1985 Jul;30(7):650–654. doi: 10.1007/BF01308414. [DOI] [PubMed] [Google Scholar]
- Bazzoli F., Roda A., Fromm H., Sarva R. P., Roda E., Barbara L. Relationship between serum and biliary bile acids as an indicator of chenodeoxycholic and ursodeoxycholic acid-induced hepatotoxicity in the rhesus monkey. Dig Dis Sci. 1982 May;27(5):417–424. doi: 10.1007/BF01295650. [DOI] [PubMed] [Google Scholar]
- Bergmann J. F., Chassany O., Guillausseau P. J., Bayle M., Chagnon S., Caulin C., Sallenave J. R. Simultaneous noninvasive evaluation of gastric emptying and orocaecal transit times. Use in studying the actions of cisapride in diabetic patients. Eur J Clin Pharmacol. 1992;43(2):121–124. doi: 10.1007/BF01740656. [DOI] [PubMed] [Google Scholar]
- Berr F., Kullak-Ublick G. A., Paumgartner G., Münzing W., Hylemon P. B. 7 alpha-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology. 1996 Dec;111(6):1611–1620. doi: 10.1016/s0016-5085(96)70024-0. [DOI] [PubMed] [Google Scholar]
- Bond J. H., Jr, Levitt M. D., Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975 Apr;85(4):546–555. [PubMed] [Google Scholar]
- Bouras E. P., Camilleri M., Burton D. D., McKinzie S. Selective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humans. Gut. 1999 May;44(5):682–686. doi: 10.1136/gut.44.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bran S., Murray W. A., Hirsch I. B., Palmer J. P. Long QT syndrome during high-dose cisapride. Arch Intern Med. 1995 Apr 10;155(7):765–768. [PubMed] [Google Scholar]
- Camilleri M., Brown M. L., Malagelada J. R. Impaired transit of chyme in chronic intestinal pseudoobstruction. Correction by cisapride. Gastroenterology. 1986 Sep;91(3):619–626. doi: 10.1016/0016-5085(86)90631-1. [DOI] [PubMed] [Google Scholar]
- Cano N., Cicero F., Ranieri F., Martin J., di Costanzo J. Ultrasonographic study of gallbladder motility during total parenteral nutrition. Gastroenterology. 1986 Aug;91(2):313–317. doi: 10.1016/0016-5085(86)90562-7. [DOI] [PubMed] [Google Scholar]
- Carulli N., Loria P., Bertolotti M., Ponz de Leon M., Menozzi D., Medici G., Piccagli I. Effects of acute changes of bile acid pool composition on biliary lipid secretion. J Clin Invest. 1984 Aug;74(2):614–624. doi: 10.1172/JCI111459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catnach S. M., Anderson J. V., Fairclough P. D., Trembath R. C., Wilson P. A., Parker E., Besser G. M., Wass J. A. Effect of octreotide on gall stone prevalence and gall bladder motility in acromegaly. Gut. 1993 Feb;34(2):270–273. doi: 10.1136/gut.34.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catnach S. M., Fairclough P. D., Trembath R. C., O'Donnell L. J., McLean A. M., Law P. A., Wickham J. E. Effect of oral erythromycin on gallbladder motility in normal subjects and subjects with gallstones. Gastroenterology. 1992 Jun;102(6):2071–2076. doi: 10.1016/0016-5085(92)90334-u. [DOI] [PubMed] [Google Scholar]
- Collins J., Goldfischer S. Perinatal hemochromatosis: one disease, several diseases or a spectrum? Hepatology. 1990 Jul;12(1):176–177. doi: 10.1002/hep.1840120131. [DOI] [PubMed] [Google Scholar]
- Dodds W. J., Groh W. J., Darweesh R. M., Lawson T. L., Kishk S. M., Kern M. K. Sonographic measurement of gallbladder volume. AJR Am J Roentgenol. 1985 Nov;145(5):1009–1011. doi: 10.2214/ajr.145.5.1009. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Hussaini S. H., Murphy G. M., Besser G. M., Wass J. A. Gallstones during octreotide therapy. Metabolism. 1992 Sep;41(9 Suppl 2):22–33. doi: 10.1016/0026-0495(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Veysey M. J., Pereira S. P., Hussaini S. H., Thomas L. A., Wass J. A., Murphy G. M. Role of intestinal transit in the pathogenesis of gallbladder stones. Can J Gastroenterol. 1997 Jan-Feb;11(1):57–64. doi: 10.1155/1997/532036. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Holden S., Brown C., Read N. W. Effect of cisapride on the gastrointestinal transit of a solid meal in normal human subjects. Gut. 1987 Jan;28(1):13–16. doi: 10.1136/gut.28.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oufir L., Flourié B., Bruley des Varannes S., Barry J. L., Cloarec D., Bornet F., Galmiche J. P. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996 Jun;38(6):870–877. doi: 10.1136/gut.38.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuessl H. S., Carolan G., Williams G., Bloom S. R. Effect of a long-acting somatostatin analogue (SMS 201-995) on postprandial gastric emptying of 99mTc-tin colloid and mouth-to-caecum transit time in man. Digestion. 1987;36(2):101–107. doi: 10.1159/000199407. [DOI] [PubMed] [Google Scholar]
- Geders J. M., Gaing A., Bauman W. A., Korsten M. A. The effect of cisapride on segmental colonic transit time in patients with spinal cord injury. Am J Gastroenterol. 1995 Feb;90(2):285–289. [PubMed] [Google Scholar]
- Goto J., Watanabe K., Miura H., Nambara T., Iida T. Studies on steroids. CCXXVIII Trace analysis of bile acids by gas chromatography-mass spectrometry with negative ion chemical ionization detection. J Chromatogr. 1987 Feb 13;388(2):379–387. doi: 10.1016/s0021-9673(01)94498-x. [DOI] [PubMed] [Google Scholar]
- Hasse C., Zielke A., Nies C., al-Bazaz B., Gotzen L., Rothmund M. Influence of ceruletid on gallbladder contraction: a possible prophylaxis of acute acalculous cholecystitis in intensive care patients? Digestion. 1995;56(5):389–394. doi: 10.1159/000201264. [DOI] [PubMed] [Google Scholar]
- Holzbach R. T. Gallbladder stasis: consequence of long-term parenteral hyperalimentation and risk factor for cholelithiasis. Gastroenterology. 1983 May;84(5 Pt 1):1055–1058. [PubMed] [Google Scholar]
- Hussaini S. H., Murphy G. M., Kennedy C., Besser G. M., Wass J. A., Dowling R. H. The role of bile composition and physical chemistry in the pathogenesis of octreotide-associated gallbladder stones. Gastroenterology. 1994 Nov;107(5):1503–1513. doi: 10.1016/0016-5085(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Hussaini S. H., Pereira S. P., Murphy G. M., Dowling R. H. Deoxycholic acid influences cholesterol solubilization and microcrystal nucleation time in gallbladder bile. Hepatology. 1995 Dec;22(6):1735–1744. [PubMed] [Google Scholar]
- Hussaini S. H., Pereira S. P., Veysey M. J., Kennedy C., Jenkins P., Murphy G. M., Wass J. A., Dowling R. H. Roles of gall bladder emptying and intestinal transit in the pathogenesis of octreotide induced gall bladder stones. Gut. 1996 May;38(5):775–783. doi: 10.1136/gut.38.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihasz M., Griffith C. A. Gallstones after vagotomy. Am J Surg. 1981 Jan;141(1):48–50. doi: 10.1016/0002-9610(81)90010-6. [DOI] [PubMed] [Google Scholar]
- Jost W. H., Schimrigk K. The effect of cisapride on delayed colonic transit time in patients with idiopathic Parkinson's disease. Wien Klin Wochenschr. 1994;106(21):673–676. [PubMed] [Google Scholar]
- Kawagishi T., Nishizawa Y., Okuno Y., Sekiya K., Morii H. Segmental gut transit in diabetes mellitus: effect of cisapride. Diabetes Res Clin Pract. 1992 Aug;17(2):137–144. doi: 10.1016/0168-8227(92)90159-o. [DOI] [PubMed] [Google Scholar]
- Kawamura O., Sekiguchi T., Itoh Z., Omura S. Effect of erythromycin derivative EM523L on human interdigestive gastrointestinal tract. Dig Dis Sci. 1993 Jun;38(6):1026–1031. doi: 10.1007/BF01295717. [DOI] [PubMed] [Google Scholar]
- Krevsky B., Malmud L. S., Maurer A. H., Somers M. B., Siegel J. A., Fisher R. S. The effect of oral cisapride on colonic transit. Aliment Pharmacol Ther. 1987 Aug;1(4):293–304. doi: 10.1111/j.1365-2036.1987.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Krevsky B., Maurer A. H., Malmud L. S., Fisher R. S. Cisapride accelerates colonic transit in constipated patients with colonic inertia. Am J Gastroenterol. 1989 Aug;84(8):882–887. [PubMed] [Google Scholar]
- Lewis S. J., Heaton K. W. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut. 1997 Aug;41(2):245–251. doi: 10.1136/gut.41.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor K. D., Lacerda M. A., Jorgensen R. A., DeSotel C. K., Batta A. K., Salen G., Dickson E. R., Rossi S. S., Hofmann A. F. Relationship between biliary and serum bile acids and response to ursodeoxycholic acid in patients with primary biliary cirrhosis. Am J Gastroenterol. 1998 Sep;93(9):1498–1504. doi: 10.1111/j.1572-0241.1998.00470.x. [DOI] [PubMed] [Google Scholar]
- Lirussi F., Vaja S., Murphy G. M., Dowling R. H. Cholestasis of total parenteral nutrition: bile acid and bile lipid metabolism in parenterally nourished rats. Gastroenterology. 1989 Feb;96(2 Pt 1):493–502. doi: 10.1016/0016-5085(89)91576-x. [DOI] [PubMed] [Google Scholar]
- Marcus S. N., Heaton K. W. Deoxycholic acid and the pathogenesis of gall stones. Gut. 1988 Apr;29(4):522–533. doi: 10.1136/gut.29.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. N., Heaton K. W. Intestinal transit, deoxycholic acid and the cholesterol saturation of bile--three inter-related factors. Gut. 1986 May;27(5):550–558. doi: 10.1136/gut.27.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzio L., DiFelice F., Laico M. G., Imbimbo B., Lapenna D., Cuccurullo F. Gallbladder hypokinesia and normal gastric emptying of liquids in patients with dyspeptic symptoms. A double-blind placebo-controlled clinical trial with cisapride. Dig Dis Sci. 1992 Feb;37(2):262–267. doi: 10.1007/BF01308181. [DOI] [PubMed] [Google Scholar]
- Masclee A. A., Jansen J. B., Driessen W. M., Geuskens L. M., Lamers C. B. Effect of truncal vagotomy on cholecystokinin release, gallbladder contraction, and gallbladder sensitivity to cholecystokinin in humans. Gastroenterology. 1990 May;98(5 Pt 1):1338–1344. doi: 10.1016/0016-5085(90)90354-4. [DOI] [PubMed] [Google Scholar]
- Metcalf A. M., Phillips S. F., Zinsmeister A. R., MacCarty R. L., Beart R. W., Wolff B. G. Simplified assessment of segmental colonic transit. Gastroenterology. 1987 Jan;92(1):40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- Meyer B. M., Werth B. A., Beglinger C., Hildebrand P., Jansen J. B., Zach D., Rovati L. C., Stalder G. A. Role of cholecystokinin in regulation of gastrointestinal motor functions. Lancet. 1989 Jul 1;2(8653):12–15. doi: 10.1016/s0140-6736(89)90255-9. [DOI] [PubMed] [Google Scholar]
- Murray F. E., Stinchcombe S. J., Hawkey C. J. Development of biliary sludge in patients on intensive care unit: results of a prospective ultrasonographic study. Gut. 1992 Aug;33(8):1123–1125. doi: 10.1136/gut.33.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagengast F. M., van Munster I. P., Salemans J. M., Van der Werf S. D. Deoxycholic acid metabolism in patients with adenomas. Gastroenterology. 1993 Sep;105(3):955–956. doi: 10.1016/0016-5085(93)90930-b. [DOI] [PubMed] [Google Scholar]
- Newman C. B., Melmed S., Snyder P. J., Young W. F., Boyajy L. D., Levy R., Stewart W. N., Klibanski A., Molitch M. E., Gagel R. F. Safety and efficacy of long-term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients--a clinical research center study. J Clin Endocrinol Metab. 1995 Sep;80(9):2768–2775. doi: 10.1210/jcem.80.9.7673422. [DOI] [PubMed] [Google Scholar]
- O'Donnell L. J., Watson A. J., Cameron D., Farthing M. J. Effect of octreotide on mouth-to-caecum transit time in healthy subjects and in the irritable bowel syndrome. Aliment Pharmacol Ther. 1990 Apr;4(2):177–181. doi: 10.1111/j.1365-2036.1990.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Patankar R., Ozmen M. M., Sanderson A., Johnson C. D. Effect of cisapride on gallbladder emptying and plasma CCK in normal and vagotomized human subjects. Dig Dis Sci. 1996 Mar;41(3):543–548. doi: 10.1007/BF02282336. [DOI] [PubMed] [Google Scholar]
- Pitt H. A., King W., 3rd, Mann L. L., Roslyn J. J., Berquist W. E., Ament M. E., DenBesten L. Increased risk of cholelithiasis with prolonged total parenteral nutrition. Am J Surg. 1983 Jan;145(1):106–112. doi: 10.1016/0002-9610(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Poen A. C., Felt-Bersma R. J., Van Dongen P. A., Meuwissen S. G. Effect of prucalopride, a new enterokinetic agent, on gastrointestinal transit and anorectal function in healthy volunteers. Aliment Pharmacol Ther. 1999 Nov;13(11):1493–1497. doi: 10.1046/j.1365-2036.1999.00629.x. [DOI] [PubMed] [Google Scholar]
- Pomare E. W., Heaton K. W., Low-Beer T. S., Espiner H. J. The effect of wheat bran upon bile salt metabolism and upon the lipid composition of bile in gallstone patients. Am J Dig Dis. 1976 Jul;21(7):521–526. doi: 10.1007/BF01464757. [DOI] [PubMed] [Google Scholar]
- Prather C. M., Camilleri M., Zinsmeister A. R., McKinzie S., Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000 Mar;118(3):463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- Quigley E. M., Marsh M. N., Shaffer J. L., Markin R. S. Hepatobiliary complications of total parenteral nutrition. Gastroenterology. 1993 Jan;104(1):286–301. doi: 10.1016/0016-5085(93)90864-9. [DOI] [PubMed] [Google Scholar]
- Rajendran S. K., Reiser J. R., Bauman W., Zhang R. L., Gordon S. K., Korsten M. A. Gastrointestinal transit after spinal cord injury: effect of cisapride. Am J Gastroenterol. 1992 Nov;87(11):1614–1617. [PubMed] [Google Scholar]
- Redfern J. S., Fortuner W. J., 2nd Octreotide-associated biliary tract dysfunction and gallstone formation: pathophysiology and management. Am J Gastroenterol. 1995 Jul;90(7):1042–1052. [PubMed] [Google Scholar]
- Setchell K. D., Matsui A. Serum bile acid analysis. Clin Chim Acta. 1983 Jan 7;127(1):1–17. doi: 10.1016/0009-8981(83)90070-0. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Worthington J. A rapid method for the quantitative extraction of bile acids and their conjugates from serum using commercially available reverse-phase octadecylsilane bonded silica cartridges. Clin Chim Acta. 1982 Oct 27;125(2):135–144. doi: 10.1016/0009-8981(82)90190-5. [DOI] [PubMed] [Google Scholar]
- Shoda J., He B. F., Tanaka N., Matsuzaki Y., Osuga T., Yamamori S., Miyazaki H., Sjövall J. Increase of deoxycholate in supersaturated bile of patients with cholesterol gallstone disease and its correlation with de novo syntheses of cholesterol and bile acids in liver, gallbladder emptying, and small intestinal transit. Hepatology. 1995 May;21(5):1291–1302. [PubMed] [Google Scholar]
- Stellaard F., Langelaar S. A., Kok R. M., Jakobs C. Determination of plasma bile acids by capillary gas-liquid chromatography-electron capture negative chemical ionization mass fragmentography. J Lipid Res. 1989 Oct;30(10):1647–1652. [PubMed] [Google Scholar]
- Stellaard F., Paumgartner G., van Berge Henegouwen G. P., van der Werf S. D. Determination of deoxycholic acid pool size and input rate using [24-13C]deoxycholic acid and serum sampling. J Lipid Res. 1986 Nov;27(11):1222–1225. [PubMed] [Google Scholar]
- Takaoka M., Kubota Y., Fujimura K., Ogura M., Kin H., Yamamoto S., Inoue K. Effect of single and multiple administrations of cisapride on postprandial gallbladder emptying in healthy humans. Intern Med. 1994 Jul;33(7):381–386. doi: 10.2169/internalmedicine.33.381. [DOI] [PubMed] [Google Scholar]
- Thomas L. A., Veysey M. J., Bathgate T., King A., French G., Smeeton N. C., Murphy G. M., Dowling R. H. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000 Sep;119(3):806–815. doi: 10.1053/gast.2000.16495. [DOI] [PubMed] [Google Scholar]
- Thorens J., Schnegg J. F., Brignoli R., Froehlich F., Jansen J. B., Dorta G., Blum A. L., Gonvers J. J., Fried M. Effect of cisapride on gallbladder motility after extracorporeal shock-wave lithotripsy. J Hepatol. 1995 Mar;22(3):333–337. doi: 10.1016/0168-8278(95)80287-8. [DOI] [PubMed] [Google Scholar]
- Thornton J. R., Heaton K. W. Do colonic bacteria contribute to cholesterol gall-stone formation? Effects of lactulose on bile. Br Med J (Clin Res Ed) 1981 Mar 28;282(6269):1018–1020. doi: 10.1136/bmj.282.6269.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollesson P. O., Cassuto J., Rimbäck G., Faxén A., Bergman L., Mattsson E. Treatment of postoperative paralytic ileus with cisapride. Scand J Gastroenterol. 1991 May;26(5):477–482. doi: 10.3109/00365529108998569. [DOI] [PubMed] [Google Scholar]
- Tonini M., De Ponti F., Di Nucci A., Crema F. Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment Pharmacol Ther. 1999 Dec;13(12):1585–1591. doi: 10.1046/j.1365-2036.1999.00655.x. [DOI] [PubMed] [Google Scholar]
- Veysey M. J., Thomas L. A., Mallet A. I., Jenkins P. J., Besser G. M., Wass J. A., Murphy G. M., Dowling R. H. Prolonged large bowel transit increases serum deoxycholic acid: a risk factor for octreotide induced gallstones. Gut. 1999 May;44(5):675–681. doi: 10.1136/gut.44.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F. H., Jr, Richards R. D., McCallum R. W. Erythromycin: a motilin agonist and gastrointestinal prokinetic agent. Am J Gastroenterol. 1993 Apr;88(4):485–490. [PubMed] [Google Scholar]
- Wiseman L. R., Faulds D. Cisapride. An updated review of its pharmacology and therapeutic efficacy as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1994 Jan;47(1):116–152. doi: 10.2165/00003495-199447010-00008. [DOI] [PubMed] [Google Scholar]
- Xu Q. W., Scott R. B., Tan D. T., Shaffer E. A. Effect of the prokinetic agent, erythromycin, in the Richardson ground squirrel model of cholesterol gallstone disease. Hepatology. 1998 Sep;28(3):613–619. doi: 10.1002/hep.510280302. [DOI] [PubMed] [Google Scholar]
- Xu Q. W., Scott R. B., Tan D. T., Shaffer E. A. Slow intestinal transit: a motor disorder contributing to cholesterol gallstone formation in the ground squirrel. Hepatology. 1996 Jun;23(6):1664–1672. doi: 10.1002/hep.510230650. [DOI] [PubMed] [Google Scholar]
- Xu Q. W., Shaffer E. A. Cisapride improves gallbladder contractility and bile lipid composition in an animal model of gallstone disease. Gastroenterology. 1993 Oct;105(4):1184–1191. doi: 10.1016/0016-5085(93)90966-g. [DOI] [PubMed] [Google Scholar]
- van Liessum P. A., Hopman W. P., Pieters G. F., Jansen J. B., Smals A. G., Rosenbusch G., Kloppenborg P. W., Lamers C. B. Postprandial gallbladder motility during long term treatment with the long-acting somatostatin analog SMS 201-995 in acromegaly. J Clin Endocrinol Metab. 1989 Sep;69(3):557–562. doi: 10.1210/jcem-69-3-557. [DOI] [PubMed] [Google Scholar]
- van de Meeberg P. C., Wolfhagen F. H., Van Berge-Henegouwen G. P., Salemans J. M., Tangerman A., van Buuren H. R., van Hattum J., van Erpecum K. J. Single or multiple dose ursodeoxycholic acid for cholestatic liver disease: biliary enrichment and biochemical response. J Hepatol. 1996 Dec;25(6):887–894. doi: 10.1016/s0168-8278(96)80293-5. [DOI] [PubMed] [Google Scholar]