Abstract
BACKGROUND—The mechanisms involved in the initiation and maintenance of Crohn's disease are poorly understood. Previous studies have demonstrated an increased number of infiltrating CD4+ T cells within the inflammatory affected bowel wall in Crohn's disease. Novel therapy approaches using anti-CD4 antibodies are thought to be effective in Crohn's disease. AIMS—Interleukin 16 (IL-16) has been characterised as a chemokine with selective chemoattraction for CD4+ inflammatory T cells. In this study, cellular expression of IL-16 in Crohn's disease and ulcerative colitis was investigated. METHODS—Expression of IL-16 was analysed in tissue samples of Crohn's disease, ulcerative colitis, and normal controls by applying reverse transcription-polymerase chain reaction, non-radioactive in situ hybridisation, and immunohistochemistry. Double staining methods were used to characterise cells expressing IL-16. The amount of infiltrating CD4+ cells was determined by immunohistochemistry and correlated with the corresponding IL-16+ cell number by step sections. RESULTS—An increased number of IL-16+ cells in Crohn's disease in comparison with ulcerative colitis and control probes was demonstrated. IL-16 was expressed by CD4 and CD8 positive T cells. In addition, in active Crohn's disease there was a substantial number of IL-16 positive mast cells. The increased number of CD4+ lymphocytes correlated positively with the increased number of IL-16 positive cells in Crohn's disease. CONCLUSION—Our results demonstrate that increased expression of IL-16 in T cells and mast cells in active Crohn's disease is associated with increased numbers of CD4+ lymphocytes. Local expression of IL-16 seems to play a significant role in the initiation and persistence of the inflammatory process in Crohn's disease, presumably by IL-16 mediated recruitment of CD4+ cells, mostly lymphocytes, into the bowel wall. Keywords: Crohn's disease; interleukin 16; inflammation; chemotaxis
Full Text
The Full Text of this article is available as a PDF (241.4 KB).
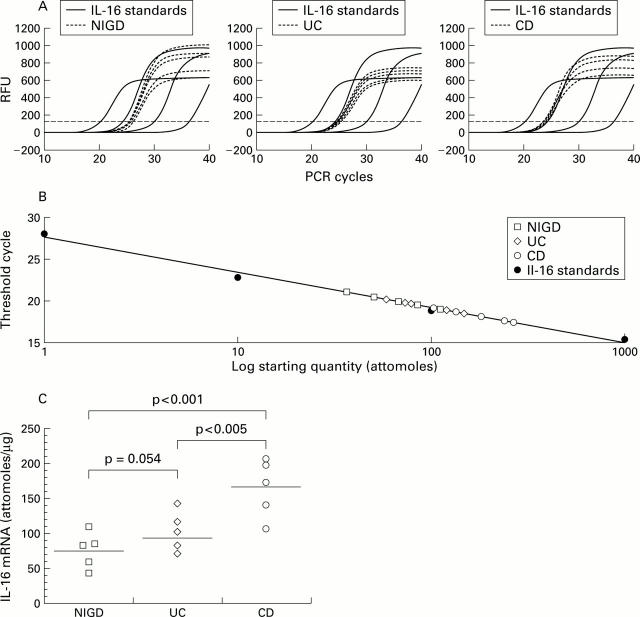
Figure 1 .
Quantitative real time reverse transcription-polymerase chain reaction (RT-PCR) analysis of interleukin 16 (IL-16) mRNA. Total RNA was analysed for IL-16 mRNA expression by quantitative RT-PCR on RNA from tissue samples affected by Crohn's disease (CD) (n=5), ulcerative colitis (UC) (n=5), and non-inflammatory gut disorder (NIGD) (n=5). The amount of IL-16 mRNA expression per µg total mRNA was derived from a standard curve using increasing amounts of in vitro generated IL-16 cRNA. Threshold cycle values of IL-16 specific amplification products were deducted from changes in SYBR Green fluorescence (RFU) during each PCR cycle for IL-16 standards and IL-16 samples from CD (A, right), UC (A, centre), and controls (A, left). The threshold (broken line) was set according to RFU variations in early PCR cycles. The calibration curve was derived from threshold cycle values (shown in A) for each known amount of IL-16 cRNA standard (1, 10, 100, and 1000 attomoles) (B). (C) In CD, significantly higher expression of IL-16 mRNA was found compared with UC (p<0.005) and NIGD (p<0.001) whereas levels of IL-16 mRNA expression in UC versus NIGD proved not to be statistically significant (p=0.054). Data are shown for one of three independent experiments.
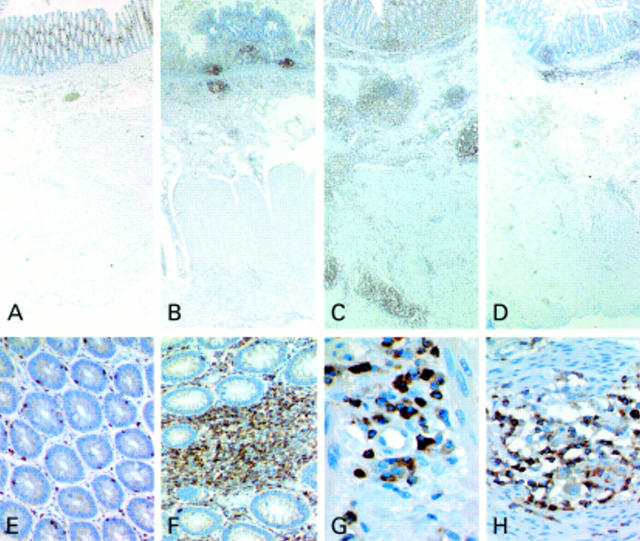
Figure 2 .
Immunohistochemistry of interleukin 16 (IL-16) protein expression. Sectional images of the bowel wall of non-inflammatory disease demonstrated that expression of IL-16 was restricted to the mucosa (A). In ulcerative colitis (UC), numerous IL-16 positive cells were found within the mucosa and within the adjacent submucosal tissue (B). In tissue specimens of active Crohn's disease (CD), numerous IL-16 positive cells were found within inflammatory aggregates of the entire bowel wall (C) whereas in sections from macroscopically uninvolved bowel of patients suffering from CD, IL-16 positive cells were predominantly restricted to the mucosa and submucosal tissue (D; A-D ×20). In non-inflammatory disorder of the large bowel, IL-16 expression was found to be restricted to a few lamina propria lymphocytes as well as to intraepithelial lymphocytes (E, ×200). In tissue specimens of CD, aggregates of lymphocytes showed strong expression of IL-16 protein in the lamina propria in addition to some intraepithelial lymphocytes (F, ×200). In submucosal layers of CD, IL-16 positive cells were predominately found in the vicinity of vessels (G, ×600). Histiocytes of CD granulomas were constantly negative for IL-16 in contrast with interspersed lymphocytes (H, ×400).
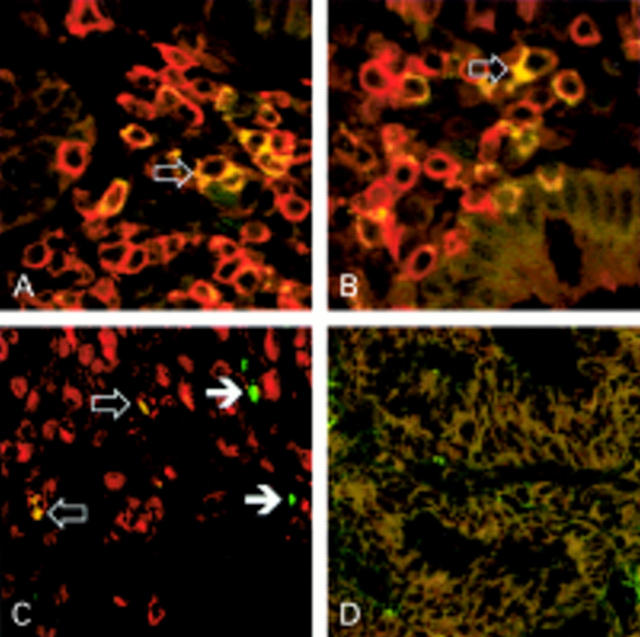
Figure 3 .
Cellular expression of interleukin 16 (IL-16) in Crohn's disease (CD). Two colour immunofluorescence was used to determine expression of IL-16 (rhodamine-red X, red fluorescence) by double labelling with antibodies for CD4 (A, ×600), CD8 (B, ×600; both green fluorescence), or mast cell specific tryptase (C; ×400, green fluorescence). Cells showing coexpression of IL-16 were visualised after digital negative colour subtraction of the single fluorescence images by a yellow colour (open arrows). Most IL-16 positive cells were found to express either CD4 (A) or CD8 (B), representing the CD3+ T cell population. Additionally, IL-16 expression was observed in mast cells (C) whereas numerous mast cells showed no coexpression of IL-16 (closed arrows). Non-specific staining was excluded by negative controls with irrelevant primary antibody (D, ×400).
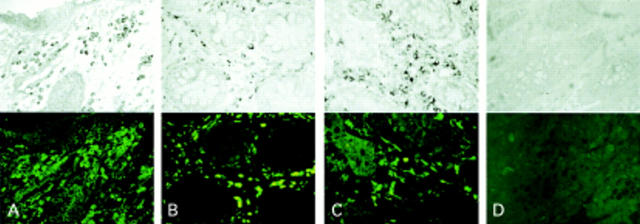
Figure 4 .
In situ interleukin 16 (IL-16) mRNA expression in Crohn's disease (CD). IL-16 mRNA was detected by non-radioactive in situ hybridisation combined with indirect immunofluorescence for the T cell marker CD3 (A, ×200), the monocyte/macrophage marker Ki-M1P (B, ×200), and S-100 protein recognising dendritic cells (C, ×200). Numerous IL-16 mRNA positive cells were found within the lamina propria in CD. The majority of IL-16 mRNA positive cells where found to be T cells showing coexpression for CD3 (A). No expression of IL-16 mRNA was observed by Ki-M1P positive macrophages (B) or S-100 protein positive dendritic cells (C). Negative controls for in situ hybridsation with sense riboprobe (D, upper, ×200) and immunofluorescence with irrelevant primary antibody (D, lower, ×200).
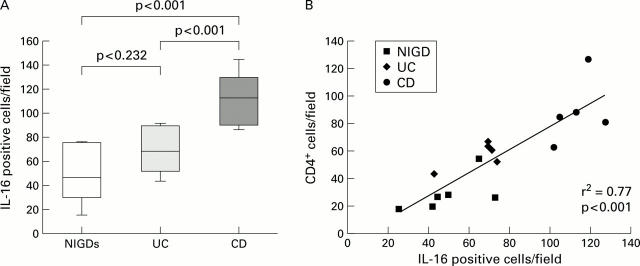
Figure 5 .
Quantitative analysis of interleukin 16 (IL-16)+ and CD4+ cell numbers in non-inflammatory gut disorder (NIGD), ulcerative colitis (UC), and Crohn's disease (CD). (A) Number of IL-16 positive cells within the lamina propria in tissue specimens from patients with NIGD, UC, and CD. Results are expressed as number of positive cells per field. IL-16 protein positive cells were significantly increased in CD compared with NIGD (p<0.001) and UC (p<0.001). No significant difference was observed between UC and NIGD (p=0.232). (B) Significant correlations between IL-16+ cells and CD4+ cell infiltration in NIGD, UC, and CD.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaschke V., Reich K., Middel P., Letschert M., Sachse F., Harwix S., Neumann C. Expression of the CD4+ cell-specific chemoattractant interleukin-16 in mycosis fungoides. J Invest Dermatol. 1999 Oct;113(4):658–663. doi: 10.1046/j.1523-1747.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- Breitschopf H., Suchanek G., Gould R. M., Colman D. R., Lassmann H. In situ hybridization with digoxigenin-labeled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol. 1992;84(6):581–587. doi: 10.1007/BF00227734. [DOI] [PubMed] [Google Scholar]
- Burgio V. L., Fais S., Boirivant M., Perrone A., Pallone F. Peripheral monocyte and naive T-cell recruitment and activation in Crohn's disease. Gastroenterology. 1995 Oct;109(4):1029–1038. doi: 10.1016/0016-5085(95)90560-x. [DOI] [PubMed] [Google Scholar]
- Center D. M., Kornfeld H., Cruikshank W. W. Interleukin 16 and its function as a CD4 ligand. Immunol Today. 1996 Oct;17(10):476–481. doi: 10.1016/0167-5699(96)10052-i. [DOI] [PubMed] [Google Scholar]
- Chupp G. L., Wright E. A., Wu D., Vallen-Mashikian M., Cruikshank W. W., Center D. M., Kornfeld H., Berman J. S. Tissue and T cell distribution of precursor and mature IL-16. J Immunol. 1998 Sep 15;161(6):3114–3119. [PubMed] [Google Scholar]
- Cruikshank W. W., Center D. M., Nisar N., Wu M., Natke B., Theodore A. C., Kornfeld H. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5109–5113. doi: 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deusch K., Reich K. Immunological aspects of inflammatory bowel disease. Endoscopy. 1992 Aug;24(6):568–577. doi: 10.1055/s-2007-1010547. [DOI] [PubMed] [Google Scholar]
- Franz J. K., Kolb S. A., Hummel K. M., Lahrtz F., Neidhart M., Aicher W. K., Pap T., Gay R. E., Fontana A., Gay S. Interleukin-16, produced by synovial fibroblasts, mediates chemoattraction for CD4+ T lymphocytes in rheumatoid arthritis. Eur J Immunol. 1998 Sep;28(9):2661–2671. doi: 10.1002/(SICI)1521-4141(199809)28:09<2661::AID-IMMU2661>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gulwani-Akolkar B., Akolkar P. N., Minassian A., Pergolizzi R., McKinley M., Mullin G., Fisher S., Silver J. Selective expansion of specific T cell receptors in the inflamed colon of Crohn's disease. J Clin Invest. 1996 Sep 15;98(6):1344–1354. doi: 10.1172/JCI118921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A., Dunzendorfer S., Offner F. A., Ryan T., Schwabegger A., Cruikshank W. W., Wiedermann C. J., Tilg H. A role for IL-16 in the cross-talk between dendritic cells and T cells. J Immunol. 1999 Sep 15;163(6):3232–3238. [PubMed] [Google Scholar]
- Keates A. C., Castagliuolo I., Cruickshank W. W., Qiu B., Arseneau K. O., Brazer W., Kelly C. P. Interleukin 16 is up-regulated in Crohn's disease and participates in TNBS colitis in mice. Gastroenterology. 2000 Oct;119(4):972–982. doi: 10.1053/gast.2000.18164. [DOI] [PubMed] [Google Scholar]
- Laberge S., Cruikshank W. W., Kornfeld H., Center D. M. Histamine-induced secretion of lymphocyte chemoattractant factor from CD8+ T cells is independent of transcription and translation. Evidence for constitutive protein synthesis and storage. J Immunol. 1995 Sep 15;155(6):2902–2910. [PubMed] [Google Scholar]
- Laberge S., Ernst P., Ghaffar O., Cruikshank W. W., Kornfeld H., Center D. M., Hamid Q. Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma. Am J Respir Cell Mol Biol. 1997 Aug;17(2):193–202. doi: 10.1165/ajrcmb.17.2.2750. [DOI] [PubMed] [Google Scholar]
- Laberge S., Ghaffar O., Boguniewicz M., Center D. M., Leung D. Y., Hamid Q. Association of increased CD4+ T-cell infiltration with increased IL-16 gene expression in atopic dermatitis. J Allergy Clin Immunol. 1998 Oct;102(4 Pt 1):645–650. doi: 10.1016/s0091-6749(98)70282-9. [DOI] [PubMed] [Google Scholar]
- Laberge S., Pinsonneault S., Ernst P., Olivenstein R., Ghaffar O., Center D. M., Hamid Q. Phenotype of IL-16-producing cells in bronchial mucosa: evidence for the human eosinophil and mast cell as cellular sources of IL-16 in asthma. Int Arch Allergy Immunol. 1999 Jun;119(2):120–125. doi: 10.1159/000024186. [DOI] [PubMed] [Google Scholar]
- Lim K. G., Wan H. C., Bozza P. T., Resnick M. B., Wong D. T., Cruikshank W. W., Kornfeld H., Center D. M., Weller P. F. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte chemoattractant factor) and RANTES. J Immunol. 1996 Apr 1;156(7):2566–2570. [PubMed] [Google Scholar]
- Lowes J. R., Jewell D. P. The immunology of inflammatory bowel disease. Springer Semin Immunopathol. 1990;12(2-3):251–268. doi: 10.1007/BF00197510. [DOI] [PubMed] [Google Scholar]
- Mekori Y. A., Metcalfe D. D. Mast cell-T cell interactions. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- Pallone F., Fais S., Squarcia O., Biancone L., Pozzilli P., Boirivant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987 Jun;28(6):745–753. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parronchi P., Romagnani P., Annunziato F., Sampognaro S., Becchio A., Giannarini L., Maggi E., Pupilli C., Tonelli F., Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997 Mar;150(3):823–832. [PMC free article] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Hansmann M. L., Peters K. P. Monoclonal antibody Ki-M4 specifically recognizes human dendritic reticulum cells (follicular dendritic cells) and their possible precursor in blood. Blood. 1983 Sep;62(3):585–590. [PubMed] [Google Scholar]
- Radzun H. J., Hansmann M. L., Heidebrecht H. J., Bödewadt-Radzun S., Wacker H. H., Kreipe H., Lumbeck H., Hernandez C., Kuhn C., Parwaresch M. R. Detection of a monocyte/macrophage differentiation antigen in routinely processed paraffin-embedded tissues by monoclonal antibody Ki-M1P. Lab Invest. 1991 Sep;65(3):306–315. [PubMed] [Google Scholar]
- Rumsaeng V., Cruikshank W. W., Foster B., Prussin C., Kirshenbaum A. S., Davis T. A., Kornfeld H., Center D. M., Metcalfe D. D. Human mast cells produce the CD4+ T lymphocyte chemoattractant factor, IL-16. J Immunol. 1997 Sep 15;159(6):2904–2910. [PubMed] [Google Scholar]
- Schluesener H. J., Seid K., Kretzschmar J., Meyermann R. Leukocyte chemotactic factor, a natural ligand to CD4, is expressed by lymphocytes and microglial cells of the MS plaque. J Neurosci Res. 1996 Jun 15;44(6):606–611. doi: 10.1002/(SICI)1097-4547(19960615)44:6<606::AID-JNR11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Stronkhorst A., Radema S., Yong S. L., Bijl H., ten Berge I. J., Tytgat G. N., van Deventer S. J. CD4 antibody treatment in patients with active Crohn's disease: a phase 1 dose finding study. Gut. 1997 Mar;40(3):320–327. doi: 10.1136/gut.40.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]