Abstract
OBJECTIVE—To analyse the morphological aspects of the extracellular matrix and microcirculation to clarify whether chronic Chagas' cardiopathy (CCC) is an accurate model to study the pathogenesis of idiopathic dilated cardiomyopathy (IDCM). DESIGN—Thick histological myocardial sections were prepared to analyse collagen, and microcirculation was examined during confocal laser and light microscopy. SETTING—The specimens were prepared at the pathology service of the Heart Institute of São Paulo, Brazil. PATIENTS—Nine control hearts, eight IDCM hearts, and 10 CCC hearts were studied after necropsy. MAIN OUTCOME MEASURES—The number of collagen struts per 100× field, the area of fibrosis (%), and the diameters of arterioles and capillaries were measured in each heart to establish outcome. RESULTS—A smaller number (mean (SD)) of collagen struts was seen in the hearts in the IDCM group (9.1 (4.1)) than in the control (22.4 (3.2)) (p < 0.05) or CCC (15.7 (7.4)) (p > 0.05) groups. Fibrosis was greater in the CCC hearts (13.8 (10.5)%) than in the IDCM hearts (5.9 (6.6)%) (p > 0.05). Major increases in arteriole (65.4 (9.9) µm) and capillary (9.9 (1.7) µm) diameters were seen in the CCC hearts but not in the IDCM hearts (arteriole diameter 40.3 (7.9) µm; capillary diameter 7.9 (1.3) µm). CONCLUSIONS—Hearts demonstrating CCC and IDCM present different extracellular and microvessel alterations. This suggests that distinct pathogenic mechanisms are responsible for each condition and that CCC is not an effective model to study IDCM. Keywords: microcirculation; Chagas' disease; dilated cardiomyopathy; extracellular matrix
Full Text
The Full Text of this article is available as a PDF (223.8 KB).
Figure 1 .
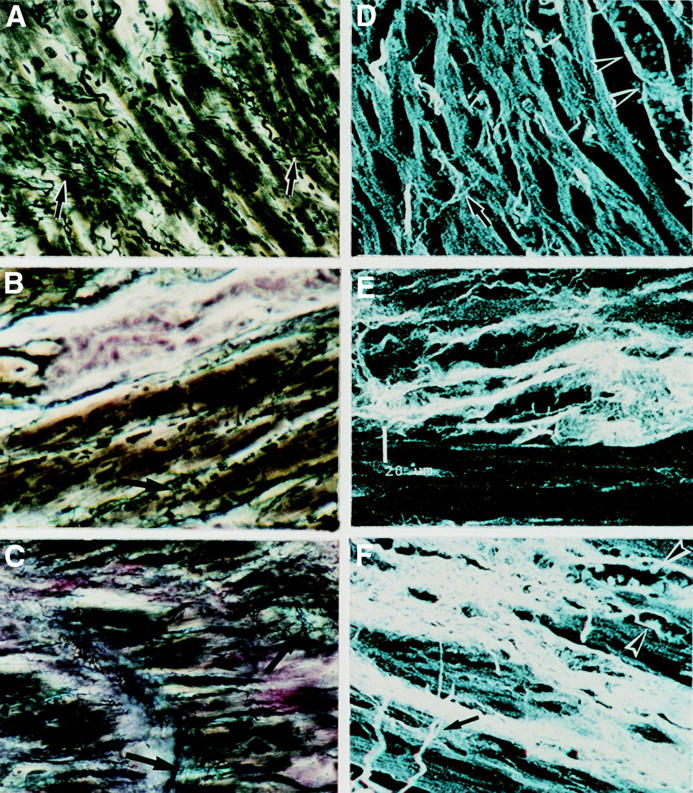
Microscopic fields of extracellular matrix in normal (A, D), IDCM (B, E) and CCC (C, F) hearts. The frames illustrate myocardium stained by the Hortega technique in a 200× microscopic field (A, B, C), and with sirius red and examined by confocal laser microscopy (D, E, F). Note the microscopic field of: (A) normal myocardium with the presence of several struts (arrows); (B) IDCM myocardium exhibiting scarce and fragmented struts (arrows) and foci of fibrosis in pink; (C) CCC myocardium exhibiting severe and diffuse fibrosis in pink enveloping the myocytes as well as some thickened struts (arrows); (D) normal myocardium exhibiting myocytes interlinked by thin collagen struts (arrow) and the arteriole running parallel to the myocytes (arrow heads); (E) IDCM myocardium showing non-compacted perivascular fibrosis; (F) CCC myocardium exhibiting severe fibrosis surrounding individual or groups of hypertrophic myocytes, with capillaries (head of arrows) distorted in the areas of fibrosis and the presence of thickened struts (arrow).
Figure 2 .
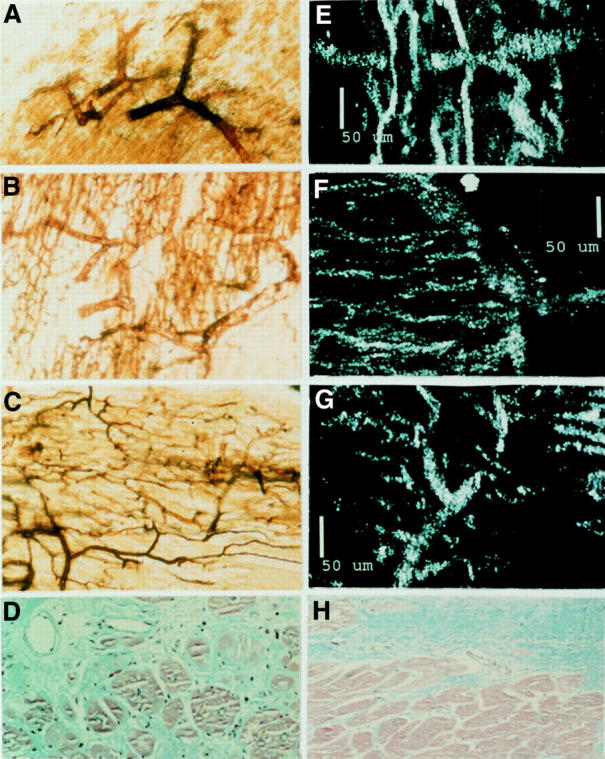
Microscopic appearance of the myocardial microcirculation in normal hearts, CCC hearts, and IDCM hearts. The 40 µm thick myocardial sections of hearts with microvessels perfused with silver solution (64×) are shown in A, B, and C: (A) normal myocardial microvessels; (B) CCC myocardium exhibiting dilated arterioles and an increased number of capillaries; (C) IDCM myocardium showing straightened arterioles and normal capillary vascularisation. Note also (D) Masson-stained CCC myocardium exhibiting diffuse fibrosis and dilated microvessels with thin and fibrotic walls. E, F, and G demonstrate corresponding reflected confocal laser microscopy views seen in A, B, and C: (E) normal microcirculation; (F) CCC microcirculation; (G) IDCM microcirculation. (H) Masson stained IDCM myocardium presenting well delimited foci of fibrosis adjacent to a non-fibrotic area of myocardium without dilated microvessels.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltrami C. A., Finato N., Rocco M., Feruglio G. A., Puricelli C., Cigola E., Sonnenblick E. H., Olivetti G., Anversa P. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995 Jan;27(1):291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- Bestetti R. B., Finzi L. A., Oliveira J. S. Chronic Chagas' heart disease presenting as an impending myocardial infarction: a case favoring the neurogenic pathogenesis concept. Clin Cardiol. 1987 Jun;10(6):368–370. doi: 10.1002/clc.4960100614. [DOI] [PubMed] [Google Scholar]
- Bishop J. E., Greenbaum R., Gibson D. G., Yacoub M., Laurent G. J. Enhanced deposition of predominantly type I collagen in myocardial disease. J Mol Cell Cardiol. 1990 Oct;22(10):1157–1165. doi: 10.1016/0022-2828(90)90079-h. [DOI] [PubMed] [Google Scholar]
- Chambers C. E., Brown K. A. Dipyridamole-induced ST segment depression during thallium-201 imaging in patients with coronary artery disease: angiographic and hemodynamic determinants. J Am Coll Cardiol. 1988 Jul;12(1):37–41. doi: 10.1016/0735-1097(88)90353-1. [DOI] [PubMed] [Google Scholar]
- Chapadeiro E. Sôbre as lesões vasculares na cardiopatia chagásica crônica. I. Relaço parede-lume das arteríolas do miocárdio. Hospital (Rio J) 1965 May;67(5):1027–1029. [PubMed] [Google Scholar]
- De Morais C. F., Higuchi M. L., Lage S. Chagas' heart disease and myocardial infarct. Incidence and report of four necropsy cases. Ann Trop Med Parasitol. 1989 Jun;83(3):207–214. doi: 10.1080/00034983.1989.11812334. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Cho S., Wittner M., Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas' disease. Am J Trop Med Hyg. 1985 Mar;34(2):246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Cho S., Wittner M., Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas' disease. Am J Trop Med Hyg. 1985 Mar;34(2):246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Tanowitz H., Wittner M., Ventura M. C. Interstitial connective tissue matrix alterations in acute murine Chagas' disease. Clin Immunol Immunopathol. 1993 Aug;68(2):147–152. doi: 10.1006/clin.1993.1111. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Murakami-Hisaichi M., Tokuda C., Kajiya F. A silver impregnation method for study of cerebral microcirculation using confocal, light, and electron microscopy. Microvasc Res. 1996 Jan;51(1):116–120. doi: 10.1006/mvre.1996.0012. [DOI] [PubMed] [Google Scholar]
- Gallo Júnior L., Morelo Filho J., Maciel B. C., Marin Neto J. A., Martins L. E., Lima Filho E. C. Functional evaluation of sympathetic and parasympathetic system in Chagas' disease using dynamic exercise. Cardiovasc Res. 1987 Dec;21(12):922–927. doi: 10.1093/cvr/21.12.922. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Ravens J. R. A metallic impregnation method for the demonstration of cerebral vascular patterns. Acta Neuropathol. 1968 Apr 8;10(3):183–188. doi: 10.1007/BF00687721. [DOI] [PubMed] [Google Scholar]
- Junqueira L. C., Cossermelli W., Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 1978 Jun;41(3):267–274. doi: 10.1679/aohc1950.41.267. [DOI] [PubMed] [Google Scholar]
- Lage S. G., Mansur A. P., Ramires J. A., da Luz P., Bellotti G., Pileggi F. Acute myocardial infarction in chronic Chagas' cardiomyopathy. Report of two cases with no obstructive coronary artery lesions. Rev Inst Med Trop Sao Paulo. 1986 Mar-Apr;28(2):131–137. doi: 10.1590/s0036-46651986000200010. [DOI] [PubMed] [Google Scholar]
- Marijianowski M. M., Teeling P., Mann J., Becker A. E. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol. 1995 May;25(6):1263–1272. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- Morris S. A., Weiss L. M., Factor S., Bilezikian J. P., Tanowitz H., Wittner M. Verapamil ameliorates clinical, pathologic and biochemical manifestations of experimental chagasic cardiomyopathy in mice. J Am Coll Cardiol. 1989 Sep;14(3):782–789. doi: 10.1016/0735-1097(89)90126-5. [DOI] [PubMed] [Google Scholar]
- Oliveira J. S., dos Santos J. C., Muccillo G., Ferreira A. L. Increased capacity of the coronary arteries in chronic Chagas' heart disease: further support for the neurogenic pathogenesis concept. Am Heart J. 1985 Feb;109(2):304–308. doi: 10.1016/0002-8703(85)90598-8. [DOI] [PubMed] [Google Scholar]
- Parodi O., De Maria R., Oltrona L., Testa R., Sambuceti G., Roghi A., Merli M., Belingheri L., Accinni R., Spinelli F. Myocardial blood flow distribution in patients with ischemic heart disease or dilated cardiomyopathy undergoing heart transplantation. Circulation. 1993 Aug;88(2):509–522. doi: 10.1161/01.cir.88.2.509. [DOI] [PubMed] [Google Scholar]
- Robinson T. F., Cohen-Gould L., Factor S. M., Eghbali M., Blumenfeld O. O. Structure and function of connective tissue in cardiac muscle: collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc. 1988 Jun;2(2):1005–1015. [PubMed] [Google Scholar]
- Robinson T. F., Factor S. M., Capasso J. M., Wittenberg B. A., Blumenfeld O. O., Seifter S. Morphology, composition, and function of struts between cardiac myocytes of rat and hamster. Cell Tissue Res. 1987 Aug;249(2):247–255. doi: 10.1007/BF00215507. [DOI] [PubMed] [Google Scholar]
- Rossi M. A., Gonçalves S., Ribeiro-dos-Santos R. Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice. The potential role of intravascular platelet aggregation in its genesis. Am J Pathol. 1984 Feb;114(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Rossi M. A., Gonçalves S., Ribeiro-dos-Santos R. Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice. The potential role of intravascular platelet aggregation in its genesis. Am J Pathol. 1984 Feb;114(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Rossi M. A. Patterns of myocardial fibrosis in idiopathic cardiomyopathies and chronic Chagasic cardiopathy. Can J Cardiol. 1991 Sep;7(7):287–294. [PubMed] [Google Scholar]
- TORRES C. M. Arterioloscerose das finas ramificaçes arterials do miocárdic (coronarite chagásica) e miocitolise focal do miocárdio na cardiopatia chagásica cronica. Hospital (Rio J) 1958 Nov;54(5):597–610. [PubMed] [Google Scholar]
- Tanowitz H. B., Burns E. R., Sinha A. K., Kahn N. N., Morris S. A., Factor S. M., Hatcher V. B., Bilezikian J. P., Baum S. G., Wittner M. Enhanced platelet adherence and aggregation in Chagas' disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990 Sep;43(3):274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Pick R., Janicki J. S., Gadodia G., Lakier J. B. Inadequate collagen tethers in dilated cardiopathy. Am Heart J. 1988 Dec;116(6 Pt 1):1641–1646. doi: 10.1016/0002-8703(88)90763-6. [DOI] [PubMed] [Google Scholar]


