Abstract
OBJECTIVE—To provide better insights into the genesis of neointimal thickening in human vein grafts early after surgery. DESIGN—Retrospective study. SETTING—Tertiary referral centre. SUBJECTS—18 distal anastomotic sites of patent grafts, obtained at necropsy from eight patients who died over differing periods (ranging from two days to nine months) after the procedure. MAIN OUTCOME MEASURES—Immunohistochemical evaluation of smooth muscle cell phenotype modulation in relation to proliferative activity. RESULTS—The earliest changes are characterised by loss of surface lining endothelial cells and insudation of blood corpuscular elements admixed with fibrin-platelet thrombus. At sites of injury vimentin positive and actin negative spindle shaped cells appear in the intima, while the related pre-existent media shows focal absence of actin positive smooth muscle cells. Proliferative activity colocalises at these sites. With time distinct neointimal thickening occurs, associated with disappearance of proliferative activity and a phenotypic shift of the smooth muscle cells. CONCLUSIONS—The observation that luminal endothelial cell denudation, with insudation of the intima with blood elements, occurs in the very early stages suggests that these phenomena are responsible for the observed dedifferentiation of pre-existent smooth muscle cells, known to be a prerequisite for cell proliferation and the evolution of intimal thickening. It is likely, therefore, that platelet released growth factors play a pivotal role, which thus may provide a target for preventive pharmacological intervention. Keywords: smooth muscle cell proliferation; vein graft stenosis; platelet derived growth factor; platelet receptor inhibitors
Full Text
The Full Text of this article is available as a PDF (205.2 KB).
Figure 1 .
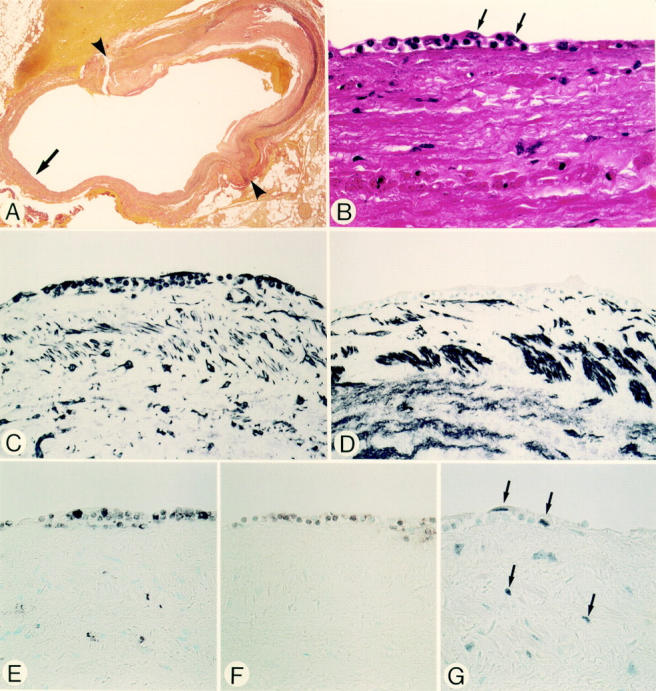
Micrographs of an anastomotic site, taken two days after grafting (patient 1). Panels A-G are serial sections. (A) Elastic tissue stain. The site of anastomosis, indicated by arrowheads, with the right coronary artery. The luminal surface of the vein graft shows a cellular response. Details of the cellular response, indicated by the arrow, are shown in panels B-G. (B) Haematoxylin and eosin stain. At the luminal surface endothelial cells have been denuded. The earliest cellular response of the grafts is demonstrated by an accumulation of polymorphonuclear leucocytes and mononuclear round cells, amid a fibrin-platelet thrombus, partially covered by spindle shaped cells (arrows). (C) Vimentin stain. Spindle shaped cells and round cells at the response site are positive. (D) HHF-35. The spindle shaped cells and round cells are negative. (E) HAM-56. Some round cells stain with this macrophage marker. None of the spindle shaped cells at the luminal site stain positive. (F) UCHL-1. Some small round cells stain with this T lymphocyte marker. (G) PCNA. Positive cells are seen at the site of cellular response and in the adjacent pre-existent media. Original magnification: (A) × 18; (B) × 580; (C-G) × 360.
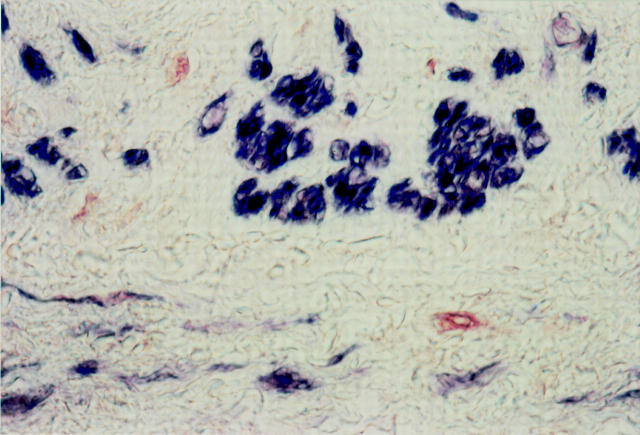
Figure 2 .
Double immunostaining with HHF-35 (blue) and PCNA (red) of an anastomotic site, two days after grafting (patient 1). The media contains a few PCNA positive cells (red), which lack staining for actin and most likely represent dedifferentiated SMCs. Most SMCs are actin positive (blue). Original magnification × 720.
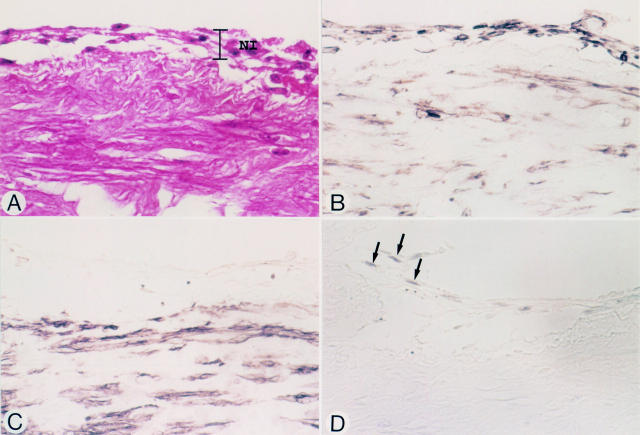
Figure 3 .
Micrographs of a vein graft at an anastomotic site, nine days after grafting (patient 5). Panels A-D represent serial sections. (A) Haematoxylin and eosin stain. Endothelial cells have been denuded. Early neointimal tissue (NI) is seen at the luminal surface of the graft. (B) Vimentin stain. Both spindle shaped cells and round cells in the neointima are positive. (C) HHF-35. The cells in the neointima do not stain. (D) PCNA. Some of the HHF-35 negative spindle shaped cells (arrows) stain positive. Original magnification × 580.
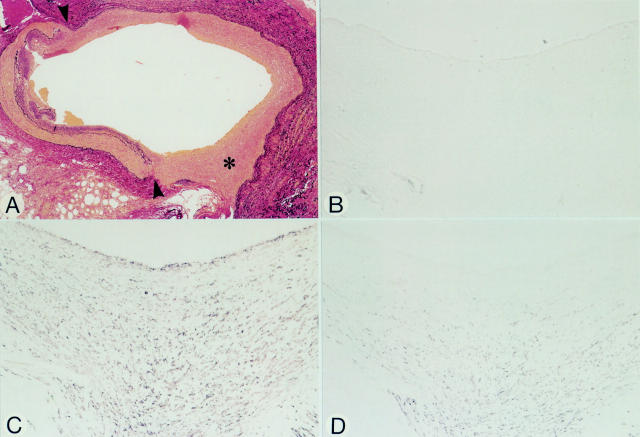
Figure 4 .
Micrographs of an anastomotic site, 33 days after grafting (patient 6). Panels A-D represent serial sections. (A) Elastic tissue stain. The anastomotic site (arrowheads) to the obtuse marginal branch shows distinct neointimal proliferation of the vein graft (asterisk). (B) HAM-56. No positivity for macrophages in the neointima. (C) HHF-35. Spindle shaped cells in the neointima stain positive. (D) CGA-7. Spindle shaped cells within the deeper layers of the neointima stain positive, but those closer to the luminal site are negative suggesting that these cells have not yet fully differentiated. The anti-vWf antibody was negative at the luminal side (not shown). Original magnification: (A) × 36; (B-D) × 145.
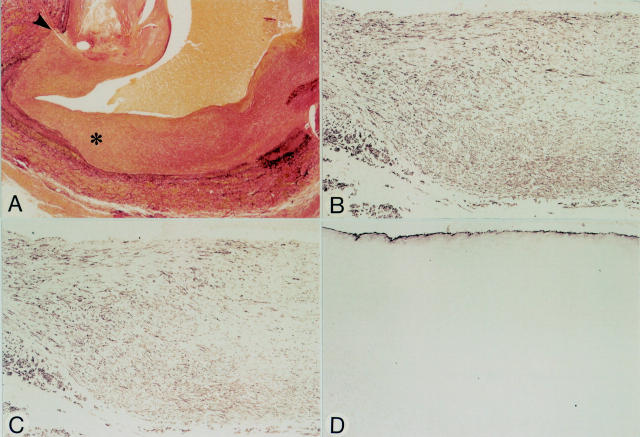
Figure 5 .
Micrographs of an anastomotic site, nine months after grafting (patient 8). Panels A-D represent serial sections. (A) Elastic tissue stain. Note distinct neointimal tissue at the anastomotic site (arrowhead). The neointima at the site of the asterisk is shown at higher magnification in panels B-D. (B) HHF-35. Spindle shaped cells in the neointima are positive. (C) CGA-7. Most spindle shaped cells are positive suggesting that most cells have the phenotype of fully differentiated SMCs (compare to panel B). (D) Anti-vWf. Regenerated endothelial cells line the luminal surface. Original magnification: (A) × 30; (B-D) × 90.
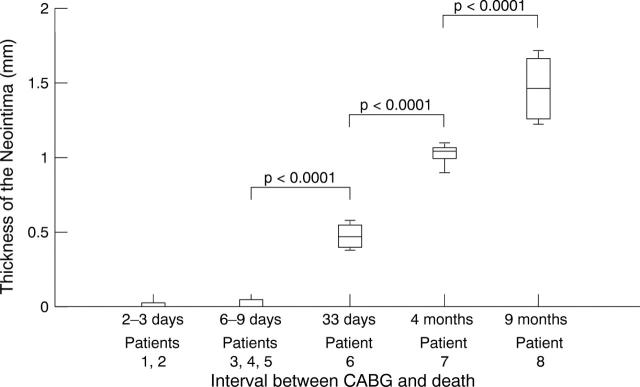
Figure 6 .
Thickness of the neointima.
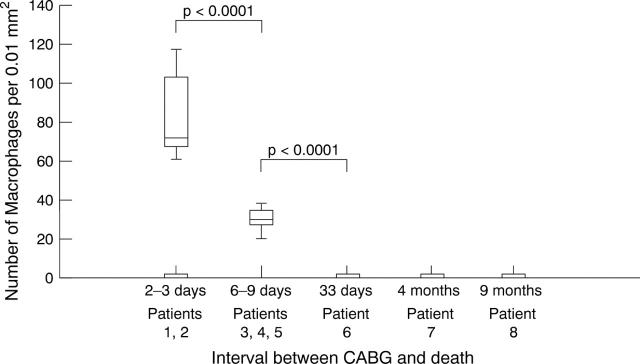
Figure 7 .
Number of macrophages within the neointima.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano J., Suzuki A., Sunamori M., Tsukada T., Numano F. Cytokinetic study of aortocoronary bypass vein grafts in place for less than six months. Am J Cardiol. 1991 Jun 1;67(15):1234–1236. doi: 10.1016/0002-9149(91)90933-c. [DOI] [PubMed] [Google Scholar]
- Arnold A. E., Simoons M. L., Van de Werf F., de Bono D. P., Lubsen J., Tijssen J. G., Serruys P. W., Verstraete M. Recombinant tissue-type plasminogen activator and immediate angioplasty in acute myocardial infarction. One-year follow-up. The European Cooperative Study Group. Circulation. 1992 Jul;86(1):111–120. doi: 10.1161/01.cir.86.1.111. [DOI] [PubMed] [Google Scholar]
- Austen W. G., Edwards J. E., Frye R. L., Gensini G. G., Gott V. L., Griffith L. S., McGoon D. C., Murphy M. L., Roe B. B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975 Apr;51(4 Suppl):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- Bai H., Masuda J., Sawa Y., Nakano S., Shirakura R., Shimazaki Y., Ogata J., Matsuda H. Neointima formation after vascular stent implantation. Spatial and chronological distribution of smooth muscle cell proliferation and phenotypic modulation. Arterioscler Thromb. 1994 Nov;14(11):1846–1853. doi: 10.1161/01.atv.14.11.1846. [DOI] [PubMed] [Google Scholar]
- Brody W. R., Angeli W. W., Kosek J. C. Histologic fate of the venous coronary artery bypass in dogs. Am J Pathol. 1972 Jan;66(1):111–130. [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. R., Stark V. K., Hullett D. A., Turnipseed W. D. Vein graft intimal hyperplasia: leukocytes and cytokine gene expression. Surgery. 1994 Aug;116(2):463–471. [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalan J. M., Roberts W. C. Morphologic findings in saphenous veins used as coronary arterial bypass conduits for longer than 1 year: necropsy analysis of 53 patients, 123 saphenous veins, and 1865 five-millimeter segments of veins. Am Heart J. 1990 May;119(5):1164–1184. doi: 10.1016/s0002-8703(05)80249-2. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani F., Gabbiani G., Reidy M. A., Cokay M. S., Peters H., Hüttner I. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest. 1991 Oct;65(4):459–470. [PubMed] [Google Scholar]
- Kockx M. M., Cambier B. A., Bortier H. E., De Meyer G. R., Van Cauwelaert P. A. The modulation of smooth muscle cell phenotype is an early event in human aorto-coronary saphenous vein grafts. Virchows Arch A Pathol Anat Histopathol. 1992;420(2):155–162. doi: 10.1007/BF02358807. [DOI] [PubMed] [Google Scholar]
- O'Brien E. R., Alpers C. E., Stewart D. K., Ferguson M., Tran N., Gordon D., Benditt E. P., Hinohara T., Simpson J. B., Schwartz S. M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993 Aug;73(2):223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Takagi M., Ueda M., Becker A. E., Takeuchi K., Takeda T. The Watanabe heritable hyperlipidemic rabbit is a suitable experimental model to study differences in tissue response between intimal and medical injury after balloon angioplasty. Arterioscler Thromb Vasc Biol. 1997 Dec;17(12):3611–3619. doi: 10.1161/01.atv.17.12.3611. [DOI] [PubMed] [Google Scholar]
- Tanizawa S., Ueda M., van der Loos C. M., van der Wal A. C., Becker A. E. Expression of platelet derived growth factor B chain and beta receptor in human coronary arteries after percutaneous transluminal coronary angioplasty: an immunohistochemical study. Heart. 1996 Jun;75(6):549–556. doi: 10.1136/hrt.75.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Kasayuki N., Kojima A., Morita Y., Tanaka S. In situ detection of platelet-derived growth factor-A and -B chain mRNA in human coronary arteries after percutaneous transluminal coronary angioplasty. Am J Pathol. 1996 Sep;149(3):831–843. [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Naruko T., Kojima A. Smooth muscle cell de-differentiation is a fundamental change preceding wound healing after percutaneous transluminal coronary angioplasty in humans. Coron Artery Dis. 1995 Jan;6(1):71–81. doi: 10.1097/00019501-199501000-00011. [DOI] [PubMed] [Google Scholar]
- Ueda M., Becker A. E., Tsukada T., Numano F., Fujimoto T. Fibrocellular tissue response after percutaneous transluminal coronary angioplasty. An immunocytochemical analysis of the cellular composition. Circulation. 1991 Apr;83(4):1327–1332. doi: 10.1161/01.cir.83.4.1327. [DOI] [PubMed] [Google Scholar]
- Unni K. K., Kottke B. A., Titus J. L., Frye R. L., Wallace R. B., Brown A. L. Pathologic changes in aortocoronary saphenous vein grafts. Am J Cardiol. 1974 Oct 3;34(5):526–532. doi: 10.1016/0002-9149(74)90122-2. [DOI] [PubMed] [Google Scholar]
- Vlodaver Z., Edwards J. E. Pathologic changes in aortic-coronary arterial saphenous vein grafts. Circulation. 1971 Oct;44(4):719–728. doi: 10.1161/01.cir.44.4.719. [DOI] [PubMed] [Google Scholar]
- van der Loos C. M., Becker A. E., van den Oord J. J. Practical suggestions for successful immunoenzyme double-staining experiments. Histochem J. 1993 Jan;25(1):1–13. doi: 10.1007/BF00161039. [DOI] [PubMed] [Google Scholar]
- van der Wal A. C., Becker A. E., Elbers J. R., Das P. K. An immunocytochemical analysis of rapidly progressive atherosclerosis in human vein grafts. Eur J Cardiothorac Surg. 1992;6(9):469–474. doi: 10.1016/1010-7940(92)90242-p. [DOI] [PubMed] [Google Scholar]