Abstract
OBJECTIVE—To measure the health related quality of life (QoL) following mitral valve replacement in childhood. DESIGN—Cross sectional study. SETTING—Tertiary referral centre. METHODS—19 patients, median age (range) 14.4 (9.7-25.4) years, were studied at a median of 7.6 (0.5-11.2) years after their most recent mitral valve replacement. General health status was measured using age specific validated questionnaires. Ten children aged between 9-15 years completed the child health related quality of life questionnaire, and for nine older patients the UK version of the short form 36 was used. Specific questions were added to the existing questionnaires to study the effect of long term anticoagulation treatment. RESULTS—All patients in the younger age group reported impaired QoL. Five rated their QoL within the range of children with chronic physical disabilities, and in the remaining five it was worse. In the older age group, all but two patients perceived their QoL as normal or near normal compared with a reference population matched for sex and age. Having regular blood tests had a negative effect on QoL in three young children, and one older patient reported impaired QoL related to taking daily warfarin tablets. CONCLUSIONS—In this small group, the effect of mitral valve replacement on QoL appears to be age specific, with more impairment in younger children. Long term anticoagulation treatment is well tolerated in most patients. Keywords: prosthetic mitral valve; heart valve replacement; child; quality of life
Full Text
The Full Text of this article is available as a PDF (106.1 KB).
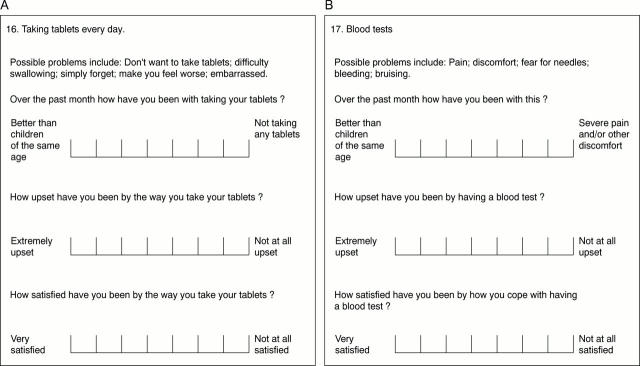
Figure 1 .
Questions added to the CQOL questionnaire to investigate the effect of taking daily tablets (A) and having regular blood tests (B). Scoring of the level of function, upset, and satisfaction was on a seven point scale, ranging from "better than children of the same age" (1 point) to "not taking any tablets" (7 points); "not at all upset" (1 point) to "extremely upset" (7 points); and "very satisfied" (1 point) to "not at all satisfied" (7 points).
Figure 2 .

Questions added to the SF-36 questionnaire to measure the effect of permanent anticoagulation on quality of life. Scoring was on a five point scale. Questions 11a and 12a: "definitely false" (1 point) to "definitely true" (5 points); the remaining questions were scored in reverse order: "definitely true" (1 point) to "definitely false" (5 points). The cumulative score for question 11 was calculated as: (a+b+c+d+e+f+g—7)/28 × 100, and for question 12 as: (a+b+c+d—4)/16 × 100. The cumulative score ranged from worst effect (0 points) to no adverse effect (100 points) on quality of life.
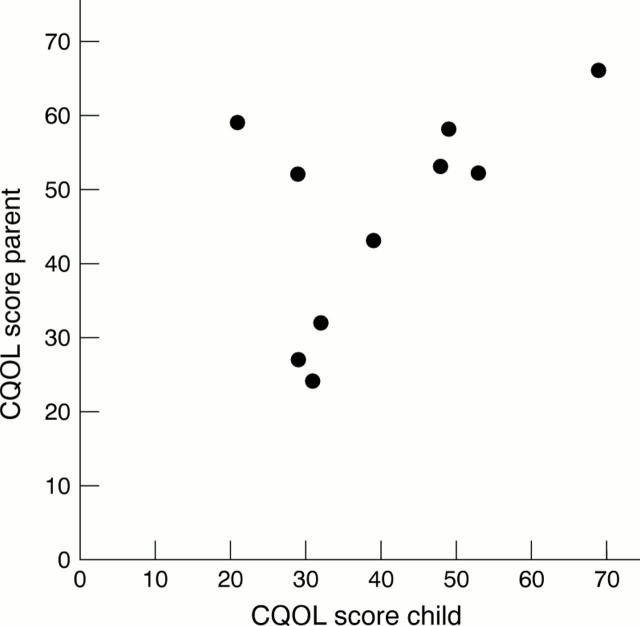
Figure 3 .
Perception of quality of life, child versus parent. In most cases the scores of the child and the parent were in good agreement. Two children scored much lower than their parents. CQOL, child health related quality of life. Lower scores indicate better quality of life.
Figure 4 .
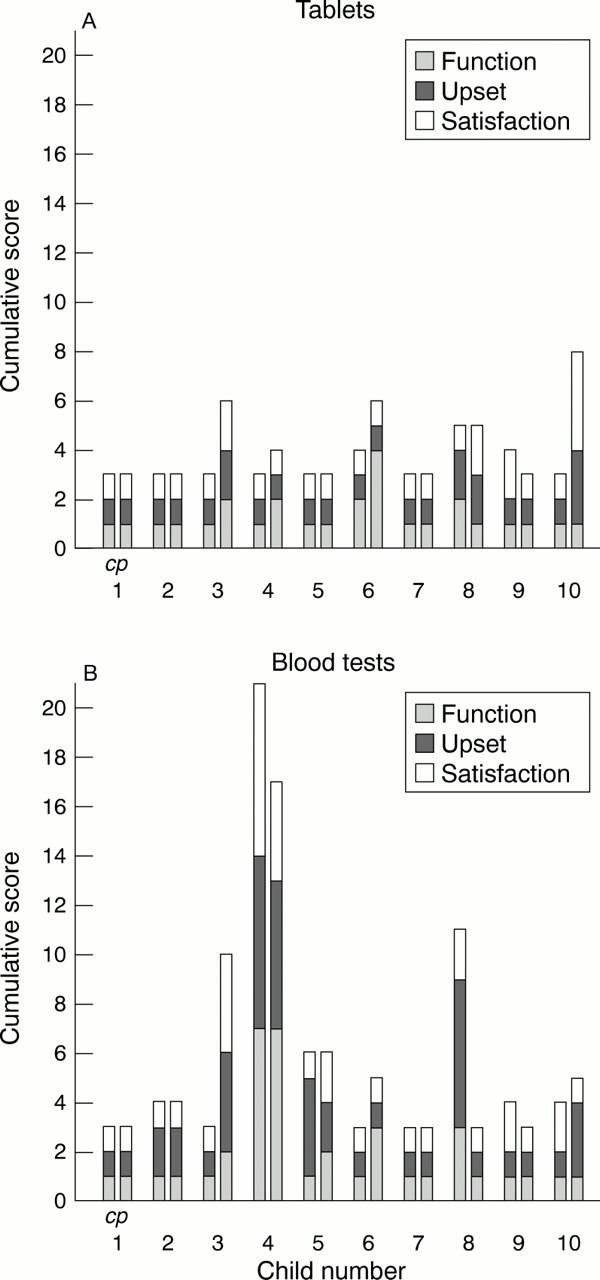
The effect of taking daily warfarin tablets (A) and having blood tests (B) on the child's quality of life as perceived by the child and parent. The level of function, upset, and satisfaction related to taking warfarin tablets and having blood tests were each scored on a scale between 1 and 7 and summed. The cumulative score ranged from 3 (no adverse effect on quality of life) to 21 (worst effect). Patient 4 suffers from needle phobia. c, child score; p, parent score for the first child and each of the following children. See text and fig 1 for detailed description of questions and scoring system.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R. S., Elliott M. J., Robinson P. J., Wyse R. K., Taylor J. F., Stark J., de Leval M. R. Surgery for congenital abnormalities of the mitral valve at the Hospital for Sick Children, London from 1969-1983. J Cardiovasc Surg (Torino) 1988 Jan-Feb;29(1):95–99. [PubMed] [Google Scholar]
- Brazier J. E., Harper R., Jones N. M., O'Cathain A., Thomas K. J., Usherwood T., Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992 Jul 18;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt A. M., Ruta D. A., Abdalla M. I., Buckingham J. K., Russell I. T. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ. 1993 May 29;306(6890):1440–1444. doi: 10.1136/bmj.306.6890.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Imai Y., Kurosawa H., Ishihara K., Kawada M., Fukuchi S. Ten-year follow-up after valve replacement with the St. Jude Medical prosthesis in children. J Thorac Cardiovasc Surg. 1990 Aug;100(2):175–180. [PubMed] [Google Scholar]
- Hasenkam J. M., Kimose H. H., Knudsen L., Grønnesby H., Halborg J., Christensen T. D., Attermann J., Pilegaard H. K. Self management of oral anticoagulant therapy after heart valve replacement. Eur J Cardiothorac Surg. 1997 May;11(5):935–942. doi: 10.1016/s1010-7940(97)01204-9. [DOI] [PubMed] [Google Scholar]
- Kadoba K., Jonas R. A., Mayer J. E., Castaneda A. R. Mitral valve replacement in the first year of life. J Thorac Cardiovasc Surg. 1990 Nov;100(5):762–768. [PubMed] [Google Scholar]
- Pal D. K. Quality of life assessment in children: a review of conceptual and methodological issues in multidimensional health status measures. J Epidemiol Community Health. 1996 Aug;50(4):391–396. doi: 10.1136/jech.50.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweng T. N., Bluett M. K., Mosca R., Callow L. B., Bove E. L. Mitral valve replacement in the first 5 years of life. Ann Thorac Surg. 1989 May;47(5):720–724. doi: 10.1016/0003-4975(89)90126-4. [DOI] [PubMed] [Google Scholar]
- van Doorn C., Yates R., Tsang V., deLeval M., Elliott M. Mitral valve replacement in children: mortality, morbidity, and haemodynamic status up to medium term follow up. Heart. 2000 Dec;84(6):636–642. doi: 10.1136/heart.84.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]