Abstract
Background/Aims—Mast cells, when activated, secrete a large number of fibrogenic factors and have been implicated in the development of fibrotic conditions of the liver, lung, and skin. There is evidence that renal fibrosis is closely linked with a chronic inflammatory cell infiltrate within the interstitium, but a potential role for mast cells in this process has yet to be defined. Therefore, the numbers of mast cells in normal and fibrotic kidneys with various pathologies were investigated.
Methods—Mast cells were quantified in renal transplants showing acute and chronic rejection and cyclosporin toxicity, kidneys removed for chronic pyelonephritis, and renal biopsies from patients with IgA nephropathy, membranous nephropathy, and diabetic nephropathy. Mast cells were stained using two methods: acid toluidine blue detected less than 30% of the mast cells revealed by immunohistochemistry for mast cell tryptase.
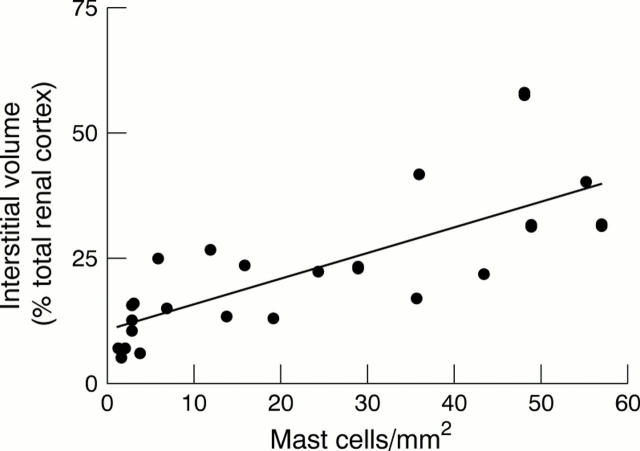
Results—Mast cells were scarce or absent in normal kidney (median, 1.6 mast cells/mm2) but numerous throughout the cortex and medulla in all specimens that showed fibrosis. They were almost entirely confined to the renal interstitium. Mast cells were present in large numbers in biopsies from patients with membranous nephropathy (median, 21.7 mast cells/mm2) and diabetic nephropathy (median, 29.2 mast cells/mm2), which were selected on the basis of showing chronic injury. In 24 unselected IgA nephropathy biopsies there was a close correlation between numbers of mast cells and the extent of interstitial fibrosis (r = 0.771; p < 0.0001). In renal transplant biopsies, mast cells were associated with allograft fibrosis in chronic rejection (median, 27.1 mast cells/mm2) and chronic cyclosporin toxicity (median, 10.6 mast cells/mm2) but not acute rejection (median, 2.7 mast cells/mm2) or acute cyclosporin toxicity (median, 2.0 mast cells/mm2). There was no detectable increase in mast cell numbers during acute rejection in those transplants that subsequently progressed to chronic rejection. In some biopsies the mast cells were largely intact, but in most cases some or all were degranulated.
Conclusions—An increased number of mast cells is a consistent feature of renal fibrosis, whatever the underlying pathology, and the number of mast cells correlates with the extent of interstitial fibrosis. This suggests that mast cells might play a pathogenetic role in the fibrotic process.
Key Words: mast cells • kidney • fibrosis
Full Text
The Full Text of this article is available as a PDF (177.9 KB).
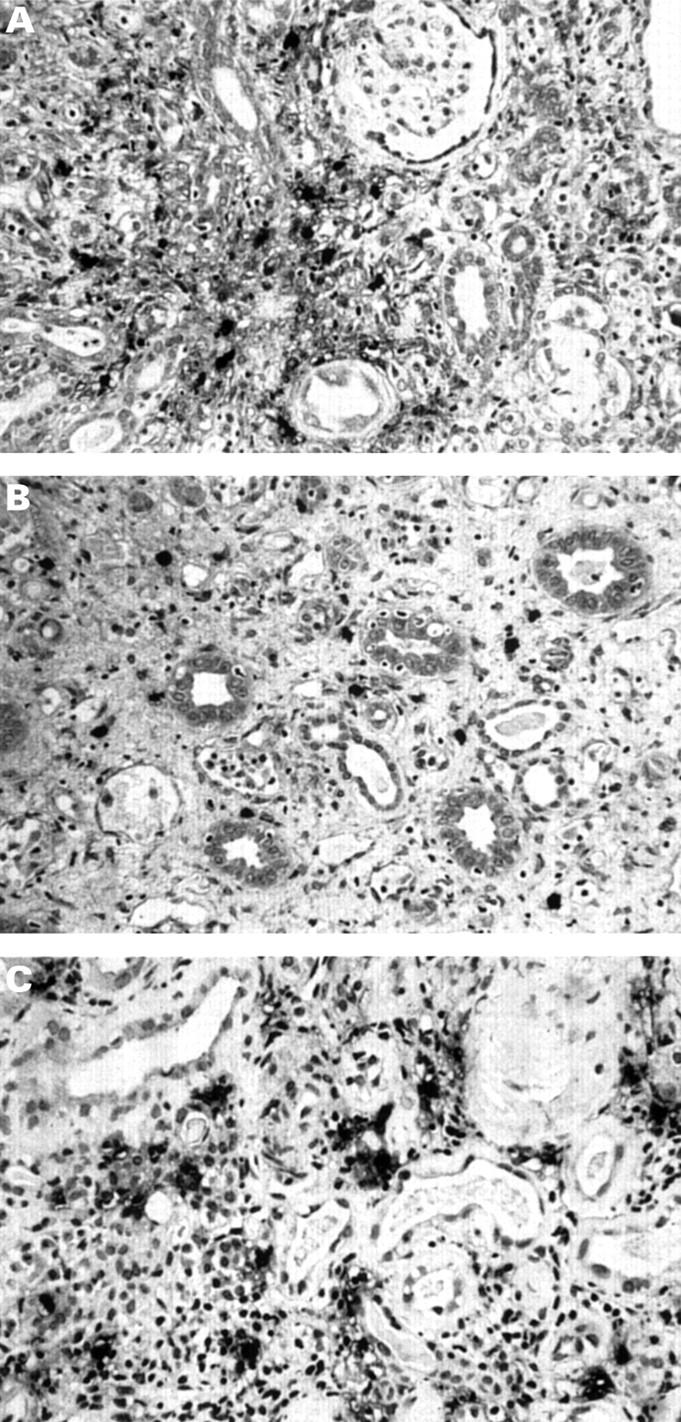
Figure 1 Mast cell infiltration in (A) the cortex and (B) the medulla in chronic renal allograft rejection. (C; IgA nephropathy) Cortical interstitial mast cells frequently show evidence of degranulation, with positivity for tryptase seen in the surrounding matrix.
Figure 2 Correlation of numbers of mast cells with cortical interstitial area, expressed as a percentage of the total renal cortex, in IgA nephropathy. Pearson's correlation: r = 0.771; p < 0.0001.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agodoa L. Y., Eggers P. W. Renal replacement therapy in the United States: data from the United States Renal Data System. Am J Kidney Dis. 1995 Jan;25(1):119–133. doi: 10.1016/0272-6386(95)90638-x. [DOI] [PubMed] [Google Scholar]
- Alexopoulos E., Seron D., Hartley R. B., Nolasco F., Cameron J. S. Immune mechanisms in idiopathic membranous nephropathy: the role of the interstitial infiltrates. Am J Kidney Dis. 1989 May;13(5):404–412. doi: 10.1016/s0272-6386(89)80024-1. [DOI] [PubMed] [Google Scholar]
- Alpers C. E., Hudkins K. L., Floege J., Johnson R. J. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol. 1994 Aug;5(2):201–209. doi: 10.1681/ASN.V52201. [DOI] [PubMed] [Google Scholar]
- Armbrust T., Batusic D., Ringe B., Ramadori G. Mast cells distribution in human liver disease and experimental rat liver fibrosis. Indications for mast cell participation in development of liver fibrosis. J Hepatol. 1997 May;26(5):1042–1054. doi: 10.1016/s0168-8278(97)80113-4. [DOI] [PubMed] [Google Scholar]
- Austin H. A., 3rd, Boumpas D. T., Vaughan E. M., Balow J. E. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int. 1994 Feb;45(2):544–550. doi: 10.1038/ki.1994.70. [DOI] [PubMed] [Google Scholar]
- Blair R. J., Meng H., Marchese M. J., Ren S., Schwartz L. B., Tonnesen M. G., Gruber B. L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997 Jun 1;99(11):2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohle A., Mackensen-Haen S., von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol. 1987;7(6):421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- Bohle A., Müller G. A., Wehrmann M., Mackensen-Haen S., Xiao J. C. Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int Suppl. 1996 May;54:S2–S9. [PubMed] [Google Scholar]
- Brenchley P. E., Short C. D., Roberts I. S. Is persistent TGFbeta1 expression the mechanism responsible for chronic renal allograft loss? Nephrol Dial Transplant. 1998 Mar;13(3):548–551. doi: 10.1093/ndt/13.3.548. [DOI] [PubMed] [Google Scholar]
- Burd P. R., Thompson W. C., Max E. E., Mills F. C. Activated mast cells produce interleukin 13. J Exp Med. 1995 Apr 1;181(4):1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. A., Walls A. F. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996 Jan 1;156(1):275–283. [PubMed] [Google Scholar]
- Cairns J. A., Walls A. F. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997 Mar 15;99(6):1313–1321. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanez P., Lacoste J. Y., Guillot B., Giron J., Barnéon G., Enander I., Godard P., Michel F. B., Bousquet J. Mast cells' contribution to the fibrosing alveolitis of the scleroderma lung. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1497–1502. doi: 10.1164/ajrccm/147.6_Pt_1.1497. [DOI] [PubMed] [Google Scholar]
- Dethlefsen S. M., Mulliken J. B., Glowacki J. An ultrastructural study of mast cell interactions in hemangiomas. Ultrastruct Pathol. 1986;10(2):175–183. doi: 10.3109/01913128609014593. [DOI] [PubMed] [Google Scholar]
- Ehara T., Shigematsu H. Contribution of mast cells to the tubulointerstitial lesions in IgA nephritis. Kidney Int. 1998 Nov;54(5):1675–1683. doi: 10.1046/j.1523-1755.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- Ferrao A. V., Mason R. M. The effect of heparin on cell proliferation and type-I collagen synthesis by adult human dermal fibroblasts. Biochim Biophys Acta. 1993 Jan 22;1180(3):225–230. doi: 10.1016/0925-4439(93)90042-y. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Floege J., Gröne H. J. Progression of renal failure: what is the role of cytokines? Nephrol Dial Transplant. 1995;10(9):1575–1586. [PubMed] [Google Scholar]
- Gershon R. K., Askenase P. W., Gershon M. D. Requirement for vasoactive amines for production of delayed-type hypersensitvity skin reactions. J Exp Med. 1975 Sep 1;142(3):732–747. doi: 10.1084/jem.142.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg G., Burnstock G. A novel cell-to-cell interaction between mast cells and other cell types. Exp Cell Res. 1983 Aug;147(1):1–13. doi: 10.1016/0014-4827(83)90265-3. [DOI] [PubMed] [Google Scholar]
- Gruber B. L., Kew R. R., Jelaska A., Marchese M. J., Garlick J., Ren S., Schwartz L. B., Korn J. H. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol. 1997 Mar 1;158(5):2310–2317. [PubMed] [Google Scholar]
- Gruber B. L., Marchese M. J., Kew R. R. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994 Jun 15;152(12):5860–5867. [PubMed] [Google Scholar]
- Gruber B. L., Marchese M. J., Kew R. Angiogenic factors stimulate mast-cell migration. Blood. 1995 Oct 1;86(7):2488–2493. [PubMed] [Google Scholar]
- Grützkau A., Krüger-Krasagakes S., Baumeister H., Schwarz C., Kögel H., Welker P., Lippert U., Henz B. M., Möller A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998 Apr;9(4):875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grützkau A., Krüger-Krasagakes S., Kögel H., Möller A., Lippert U., Henz B. M. Detection of intracellular interleukin-8 in human mast cells: flow cytometry as a guide for immunoelectron microscopy. J Histochem Cytochem. 1997 Jul;45(7):935–945. doi: 10.1177/002215549704500703. [DOI] [PubMed] [Google Scholar]
- Horton M. A., O'Brien H. A. Characterization of human mast cells in long-term culture. Blood. 1983 Dec;62(6):1251–1260. [PubMed] [Google Scholar]
- Hu Z. Q., Yamazaki T., Cai Z., Yoshida T., Shimamura T. Mast cells display natural suppressor activity partially by releasing transforming growth factor-beta. Immunology. 1994 Jul;82(3):482–486. [PMC free article] [PubMed] [Google Scholar]
- Hunt L. W., Colby T. V., Weiler D. A., Sur S., Butterfield J. H. Immunofluorescent staining for mast cells in idiopathic pulmonary fibrosis: quantification and evidence for extracellular release of mast cell tryptase. Mayo Clin Proc. 1992 Oct;67(10):941–948. doi: 10.1016/s0025-6196(12)60924-0. [DOI] [PubMed] [Google Scholar]
- Johnson D. W., Saunders H. J., Baxter R. C., Field M. J., Pollock C. A. Paracrine stimulation of human renal fibroblasts by proximal tubule cells. Kidney Int. 1998 Sep;54(3):747–757. doi: 10.1046/j.1523-1755.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Jackson C. L., Angelini G. D., George S. J. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998 Nov;18(11):1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. J., Curry A. Interaction between mast cells and perineurial fibroblasts in neurofibroma. New insights into mast cell function. Pathol Res Pract. 1988 Aug;183(4):453–461. doi: 10.1016/S0344-0338(88)80092-X. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum A. S., Goff J. P., Dreskin S. C., Irani A. M., Schwartz L. B., Metcalfe D. D. IL-3-dependent growth of basophil-like cells and mastlike cells from human bone marrow. J Immunol. 1989 Apr 1;142(7):2424–2429. [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Krüger-Krasagakes S., Möller A., Kolde G., Lippert U., Weber M., Henz B. M. Production of interleukin-6 by human mast cells and basophilic cells. J Invest Dermatol. 1996 Jan;106(1):75–79. doi: 10.1111/1523-1747.ep12327815. [DOI] [PubMed] [Google Scholar]
- Leiferman K. M., Ackerman S. J., Sampson H. A., Haugen H. S., Venencie P. Y., Gleich G. J. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med. 1985 Aug 1;313(5):282–285. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- Lyon M., Rushton G., Gallagher J. T. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J Biol Chem. 1997 Jul 18;272(29):18000–18006. doi: 10.1074/jbc.272.29.18000. [DOI] [PubMed] [Google Scholar]
- Mallick N. P., Jones E., Selwood N. The European (European Dialysis and Transplantation Association-European Renal Association) Registry. Am J Kidney Dis. 1995 Jan;25(1):176–187. doi: 10.1016/0272-6386(95)90642-8. [DOI] [PubMed] [Google Scholar]
- Meininger C. J., Zetter B. R. Mast cells and angiogenesis. Semin Cancer Biol. 1992 Apr;3(2):73–79. [PubMed] [Google Scholar]
- Ng Y. Y., Huang T. P., Yang W. C., Chen Z. P., Yang A. H., Mu W., Nikolic-Paterson D. J., Atkins R. C., Lan H. Y. Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int. 1998 Sep;54(3):864–876. doi: 10.1046/j.1523-1755.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- Norrby K. Effect of heparin, histamine and serotonin on the density-dependent inhibition of replication in two fibroblastic cell lines. Virchows Arch B Cell Pathol. 1973 Dec 31;15(1):75–93. doi: 10.1007/BF02889327. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Hirota S., Xu Z. D., Maeyama K., Nakama A., Kawano S., Hori M., Kitamura Y. Increase of mast cells in the liver and lung may be associated with but not a cause of fibrosis: demonstration using mast cell-deficient Ws/Ws rats. Lab Invest. 1998 Nov;78(11):1431–1438. [PubMed] [Google Scholar]
- Ong A. C., Fine L. G. Tubular-derived growth factors and cytokines in the pathogenesis of tubulointerstitial fibrosis: implications for human renal disease progression. Am J Kidney Dis. 1994 Feb;23(2):205–209. doi: 10.1016/s0272-6386(12)80973-5. [DOI] [PubMed] [Google Scholar]
- Pitt M. A., Roberts I. S., Curry A. Spindle cell and pleomorphic lipoma: an ultrastructural study. Ultrastruct Pathol. 1995 Nov-Dec;19(6):475–480. doi: 10.3109/01913129509014622. [DOI] [PubMed] [Google Scholar]
- Qu Z., Liebler J. M., Powers M. R., Galey T., Ahmadi P., Huang X. N., Ansel J. C., Butterfield J. H., Planck S. R., Rosenbaum J. T. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995 Sep;147(3):564–573. [PMC free article] [PubMed] [Google Scholar]
- Qu Z., Liebler J. M., Powers M. R., Galey T., Ahmadi P., Huang X. N., Ansel J. C., Butterfield J. H., Planck S. R., Rosenbaum J. T. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995 Sep;147(3):564–573. [PMC free article] [PubMed] [Google Scholar]
- Roberts I. S., Burrows C., Shanks J. H., Venning M., McWilliam L. J. Interstitial myofibroblasts: predictors of progression in membranous nephropathy. J Clin Pathol. 1997 Feb;50(2):123–127. doi: 10.1136/jcp.50.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger B. M., Hasan Q., Greenhill N. S., Davis P. F., Dunbar P. R., Neale T. J. Mast cells and type VIII collagen in human diabetic nephropathy. Diabetologia. 1996 Oct;39(10):1215–1222. doi: 10.1007/BF02658509. [DOI] [PubMed] [Google Scholar]
- Schena F. P., Gesualdo L., Grandaliano G., Montinaro V. Progression of renal damage in human glomerulonephritides: is there sleight of hand in winning the game? Kidney Int. 1997 Dec;52(6):1439–1457. doi: 10.1038/ki.1997.475. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Smith J. C., Finn M. C. The possible role of mast cells (allergy) in the production of keloid and hypertrophic scarring. J Burn Care Rehabil. 1987 Mar-Apr;8(2):126–131. doi: 10.1097/00004630-198703000-00008. [DOI] [PubMed] [Google Scholar]
- Strutz F., Neilson E. G. The role of lymphocytes in the progression of interstitial disease. Kidney Int Suppl. 1994 Feb;45:S106–S110. [PubMed] [Google Scholar]
- Taipale J., Lohi J., Saarinen J., Kovanen P. T., Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995 Mar 3;270(9):4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- Tang W. W., Van G. Y., Qi M. Myofibroblast and alpha 1 (III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int. 1997 Mar;51(3):926–931. doi: 10.1038/ki.1997.131. [DOI] [PubMed] [Google Scholar]
- Thompson H. L., Burbelo P. D., Yamada Y., Kleinman H. K., Metcalfe D. D. Mast cells chemotax to laminin with enhancement after IgE-mediated activation. J Immunol. 1989 Dec 15;143(12):4188–4192. [PubMed] [Google Scholar]
- Trautmann A., Krohne G., Bröcker E. B., Klein C. E. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol. 1998 May 15;160(10):5053–5057. [PubMed] [Google Scholar]
- Turlington B. S., Edwards W. D. Quantitation of mast cells in 100 normal and 92 diseased human hearts. Implications for interpretation of endomyocardial biopsy specimens. Am J Cardiovasc Pathol. 1988;2(2):151–157. [PubMed] [Google Scholar]
- Walker M. A., Harley R. A., LeRoy E. C. Inhibition of fibrosis in TSK mice by blocking mast cell degranulation. J Rheumatol. 1987 Apr;14(2):299–301. [PubMed] [Google Scholar]
- Wehrmann M., Bohle A., Bogenschütz O., Eissele R., Freislederer A., Ohlschlegel C., Schumm G., Batz C., Gärtner H. V. Long-term prognosis of chronic idiopathic membranous glomerulonephritis. An analysis of 334 cases with particular regard to tubulo-interstitial changes. Clin Nephrol. 1989 Feb;31(2):67–76. [PubMed] [Google Scholar]
- Wershil B. K., Murakami T., Galli S. J. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol. 1988 Apr 1;140(7):2356–2360. [PubMed] [Google Scholar]
- Wingren U., Enerbäck L. Mucosal mast cells of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan. Histochem J. 1983 Jun;15(6):571–582. doi: 10.1007/BF01954148. [DOI] [PubMed] [Google Scholar]
- Yousem S. A. The potential role of mast cells in lung allograft rejection. Hum Pathol. 1997 Feb;28(2):179–182. doi: 10.1016/s0046-8177(97)90103-9. [DOI] [PubMed] [Google Scholar]