Abstract
Aims—To investigate variation within the cag pathogenicity island (PAI) of Helicobacter pylori isolated from patients with dyspepsia in mid-Essex, and to evaluate the effect on expression of anti-CagA antibody.
Methods—Sixty two isolates of H pylori cultured from gastric biopsies were screened by specific PCR assays for the presence of cagA and other gene markers (cagD and cagE, and virD4) in the cag PAI. An enzyme linked immunosorbent assay (ELISA) kit (Viva Diagnostica helicobacter p120) was used to test for anti-CagA IgG antibody in matching sera. Isolates were also genotyped by vacuolating cytotoxin polymerase chain reaction (PCR) analysis, and tested for absence of the complete cag PAI (empty site PCR assay).
Results—Forty one of the H pylori isolates had a cag PAI containing cagA. One strain had no cagA but other cag PAI loci were present, whereas the remaining 20 strains had no detectable cag PAI markers. Anti-CagA IgG antibody was detected in 34 sera by the ELISA assay, and when compared with the cag PAI genotype of the infecting strain, accuracy, sensitivity, and specificity were 92%, 87%, and 100%, respectively. The seven discrepant or borderline strains in the ELISA were all vacA s1 but differed in other genotypic markers.
Conclusions—The cag PAI was widely distributed in H pylori from patients with dyspepsia in mid-Essex who had different gastric pathologies. Infection with a strain having an uninterrupted cag PAI was associated with the presence of anti-CagA antibody in most patients. Discrepant ELISA results, mostly for elderly patients with duodenal ulcers, were attributed to cagA associated variation, particularly to the presence of mixed cagA+/cagA- cell variants in the infecting strain population. Tests for anti-CagA serum antibody were unreliable for predicting severity of clinical disease associated with H pylori infection in this series of patients.
Key Words: Helicobacter pylori • cagA • cag pathogenicity island • CagA antibody assay
Full Text
The Full Text of this article is available as a PDF (126.1 KB).
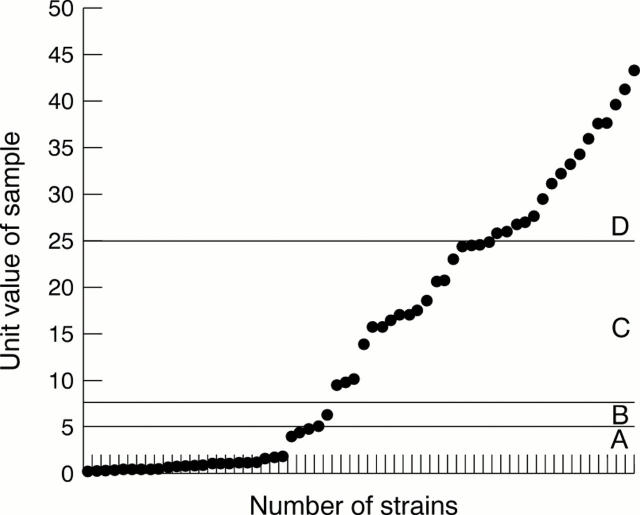
Figure 1 The range of semiquantitative values for Helicobacter pylori CagA enzyme linked immunosorbent assay. Each point represents one strain. A, negative zone; B, borderline zone; C, positive zone; and D, strong positive zone.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyants N. S., Clifton S. W., Kersulyte D., Crabtree J. E., Youree B. E., Reece C. A., Bukanov N. O., Drazek E. S., Roe B. A., Berg D. E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998 Apr;28(1):37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Cao P., Peek R. M., Jr, Tummuru M. K., Blaser M. J., Cover T. L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995 Jul 28;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Cover T. L., Twells R. J., Morales M. R., Hawkey C. J., Blaser M. J. Simple and accurate PCR-based system for typing vacuolating cytotoxin alleles of Helicobacter pylori. J Clin Microbiol. 1999 Sep;37(9):2979–2982. doi: 10.1128/jcm.37.9.2979-2982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales I. L., Crabtree J. E., Scunes D., Covacci A., Calam J. Antibodies to CagA protein are associated with gastric atrophy in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1996 Jul;8(7):645–649. [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and gastric diseases. BMJ. 1998 May 16;316(7143):1507–1510. doi: 10.1136/bmj.316.7143.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J. Not all Helicobacter pylori strains are created equal: should all be eliminated? Lancet. 1997 Apr 5;349(9057):1020–1022. doi: 10.1016/S0140-6736(96)09133-7. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Perez-Perez G. I., Kleanthous H., Cover T. L., Peek R. M., Chyou P. H., Stemmermann G. N., Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995 May 15;55(10):2111–2115. [PubMed] [Google Scholar]
- Busolo F., Bertollo G., Bordignon G., Madia D., Camposampiero D. Detection and characterization of Helicobacter pylori from patients with gastroduodenal diseases. Diagn Microbiol Infect Dis. 1998 Aug;31(4):531–536. doi: 10.1016/s0732-8893(98)00055-8. [DOI] [PubMed] [Google Scholar]
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C. K., Wong B. C., Kwok E., Ong L., Covacci A., Lam S. K. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996 May;91(5):949–953. [PubMed] [Google Scholar]
- Chow W. H., Blaser M. J., Blot W. J., Gammon M. D., Vaughan T. L., Risch H. A., Perez-Perez G. I., Schoenberg J. B., Stanford J. L., Rotterdam H. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998 Feb 15;58(4):588–590. [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A., Falkow S., Berg D. E., Rappuoli R. Did the inheritance of a pathogenicity island modify the virulence of Helicobacter pylori? Trends Microbiol. 1997 May;5(5):205–208. doi: 10.1016/S0966-842X(97)01035-4. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Glupczynski Y., Lage A. P., Burette A., Tummuru M. K., Perez-Perez G. I., Blaser M. J. Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol. 1995 Jun;33(6):1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Wyatt J. I., Sobala G. M., Miller G., Tompkins D. S., Primrose J. N., Morgan A. G. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut. 1993 Oct;34(10):1339–1343. doi: 10.1136/gut.34.10.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Cohen H., Blaser M. J. Helicobacter pylori. Clin Microbiol Rev. 1997 Oct;10(4):720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantry G. T., Zheng Q. X., Darwin P. E., Rosenstein A. H., James S. P. Mixed infection with cagA-positive and cagA-negative strains of Helicobacter pylori. Helicobacter. 1996 Jun;1(2):98–106. doi: 10.1111/j.1523-5378.1996.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Figura N., Vindigni C., Covacci A., Presenti L., Burroni D., Vernillo R., Banducci T., Roviello F., Marrelli D., Biscontri M. cagA positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: relevance to histological damage. Gut. 1998 Jun;42(6):772–778. doi: 10.1136/gut.42.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Genta R. M., Graham D. P., Crabtree J. E. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: lack of correlation of IgG antibody in patients with peptic ulcer or asymptomatic Helicobacter pylori gastritis. J Clin Pathol. 1996 Oct;49(10):829–832. doi: 10.1136/jcp.49.10.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen M., Janatuinen E., Mayo K., Mégraud F., Julkunen R., Pikkarainen P. Usefulness of anti-Helicobacter pylori and anti-CagA antibodies in the selection of patients for gastroscopy. Am J Gastroenterol. 1997 Dec;92(12):2225–2229. [PubMed] [Google Scholar]
- Jenks P. J., Mégraud F., Labigne A. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut. 1998 Dec;43(6):752–758. doi: 10.1136/gut.43.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Mitchell H. M., Hazell S. L., Li Y. Y., Hu P. J. Serological response to specific Helicobacter pylori antigens: antibody against CagA antigen is not predictive of gastric cancer in a developing country. Am J Gastroenterol. 1996 Sep;91(9):1785–1788. [PubMed] [Google Scholar]
- Owen R. J., Hurtado A., Banatvala N., Abdi Y., Davies G. R., Feldman R., Hardie J. M. Conservation of the cytotoxin-associated (cagA) gene of Helicobacter pylori and investigation of association with vacuolating-cytotoxin activity and gastroduodenal disease. FEMS Immunol Med Microbiol. 1994 Oct;9(4):307–315. doi: 10.1111/j.1574-695X.1994.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Orentreich N., Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997 Mar;40(3):297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounder R. E., Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9 (Suppl 2):33–39. [PubMed] [Google Scholar]
- Shimoyama T., Fukuda S., Tanaka M., Mikami T., Saito Y., Munakata A. High prevalence of the CagA-positive Helicobacter pylori strains in Japanese asymptomatic patients and gastric cancer patients. Scand J Gastroenterol. 1997 May;32(5):465–468. doi: 10.3109/00365529709025082. [DOI] [PubMed] [Google Scholar]
- Tomb J. F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleischmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997 Aug 7;388(6642):539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- Tummuru M. K., Cover T. L., Blaser M. J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993 May;61(5):1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995 Dec;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- Weel J. F., van der Hulst R. W., Gerrits Y., Roorda P., Feller M., Dankert J., Tytgat G. N., van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996 May;173(5):1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Graham D. Y., Kashima K. Comparison of four serological tests to determine the CagA or VacA status of Helicobacter pylori strains. J Clin Microbiol. 1998 Nov;36(11):3433–3434. doi: 10.1128/jcm.36.11.3433-3434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]