Abstract
Engagement of the T cell costimulatory receptor CTLA-4 can potently down-regulate an immune response. For example, in a T cell receptor transgenic mouse model of autoimmune diabetes, CTLA-4 interactions keep pancreatic islet-reactive T cells in check, evidenced by the finding that mAb blockade of CTLA-4 rapidly provokes diabetes in animals that would not normally succumb until many months later. Interestingly, this effect is only observed early in the course of disease, before insulitis is stably entrenched. Here, we have exploited a highly synchronous and easily manipulable transfer system to determine precisely when CTLA-4 must be engaged to check the diabetogenicity of islet-reactive T cells. Our results indicate that CTLA-4 interactions during initial priming of the T cells in the pancreatic lymph nodes are not determinant. Rather, the critical interactions occur when the T cells secondarily reencounter their antigen in the target organ, the pancreatic islets. In addition, we made use of CTLA-4-deficient mice to bolster our interpretation that CTLA-4 engagement has a dampening rather than an enhancing influence on diabetes progression.
Keywords: autoimmunity, diabetes, costimulatory molecules, T cell activation, T cell regulation
CTLA-4 (CD152) is an important regulator of the immune response (1, 2). It is a member of a growing family of costimulatory receptors specifically expressed on the surface of T cells. It and its closest homologue, CD28, bind to the B7–1 and B7–2 (CD80 and CD86, respectively) costimulatory ligands displayed primarily on antigen-presenting cells (3). Some reports have suggested that CTLA-4, like CD28, acts as a positive costimulator, transmitting a signal that somehow synergizes with the signal delivered through the T cell receptor (TCR) to promote T cell stimulation (4–6). However, the large majority of data argues for an opposing role, as a dampener of T cell activation. CTLA-4 blockade enhances T cell responses in vitro (7–16) and in vivo (16–23), augments antitumor immunity (24–26), and exacerbates autoimmune disease (27–29). In addition, mice carrying a CTLA-4-null mutation show massive accumulation of activated T cells in the peripheral lymphoid organs and leukocyte infiltration into a variety of tissues (30–32).
It is not yet clear precisely when, during an immune response, CTLA-4 must be engaged to exert its down-regulatory function. Given the difficulty of detecting it on the surface of naive T cells, and its up-regulation on T cell activation, it originally was proposed that CTLA-4 would act late in a response. There are certainly indications that this can occur (12, 28, 33–37). However, there are also examples of it acting early, to set the tone of a response (9, 10, 17, 29, 34–36, 38, 39).
We have focused on this issue by exploiting the synchrony and manipulability of a recently described transfer system (40) derived from the BDC2.5 TCR transgenic (tg) mouse model of autoimmune diabetes (41). BDC2.5 TCR tg mice carry the rearranged TCR-α and TCR-β genes from a CD4+, Th1-like, I-Ag7-restricted, islet β-cell-specific, diabetogenic T cell clone (42). When propagated on the NOD genetic background, these animals have a T cell repertoire skewed in favor of the transgene-encoded specificity and exhibit insulitis universally and abruptly between 2 and 3 weeks of age, but diabetes develops in only a fraction of individuals only months later (41, 43). Early disease events take place during a strikingly narrow time window in BDC2.5 TCR tg mice (29, 41), and they are even more synchronous in a naive BDC2.5 T cell transfer system (40). After transfer of splenocytes from juvenile BDC2.5/NOD mice into lymphocyte-deficient Cα0/0/NOD animals, it is possible to distinguish the arrival of self-reactive T cells in the lymph nodes (day 1/2–1), their activation specifically in the pancreatic lymph nodes (PLNs) (days 2–3), and their invasion of the pancreatic islets (days 5–8). This transfer system has proven very useful for dissecting factors involved in early disease processes (40).
We already have established that CTLA-4 is an important regulator of diabetes progression in the standard BDC2.5 TCR tg model (29). Injection of anti-CTLA-4 mAb into BDC2.5/NOD animals promoted an aggressive form of insulitis and rapidly provoked diabetes, but only when it was administered in juvenile animals, before there was a significant accumulation of islet infiltrate. These findings indicated that CTLA-4 engagement at a quite early stage of disease progression influences the diabetogenicity of islet-reactive T cells. The goal of the present study was to exploit the naive BDC2.5 T cell transfer system to pinpoint precisely when CTLA-4 engagement plays its determining role—during naive T cell priming in the PLNs or during secondary reencounter of antigen in the pancreatic islets.
Materials and Methods
Mice.
Mice carrying the BDC2.5 α and β TCR transgenes (41) and mice harboring a mutation in the TCR Cα locus (Cαo/o/NOD; refs. 44 and 45) have been described. These lines were backcrossed onto the NOD/Lt background for at least 20 and 12 generations, respectively. CTLA-4o/+ mice (32) carried on the C57BL/6 background were backcrossed three times to the NOD background, and mice from the third generation were intercrossed to obtain animals homozygous for the CTLA-4 null mutation. All animals were maintained in the conventional facility of the Institut de Génétique et de Biologie Moleculaire et Cellulaire, under Ministère de l'Agriculture (Agrément 67227) and European Economic Community guidelines.
Cell Transfers.
Splenocytes from 10- to 12-day-old BDC2.5/NOD mice were pooled, and the erythrocytes were lysed in 0.83% ammonium chloride; 1–2 × 107 cells were injected i.v. into adult (6–10 weeks old) Cαo/NOD mice in a total volume of 200 μl. In some experiments, cells were labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) before transfer, as described (40, 46). Briefly, cells were adjusted to 107/ml in PBS and then were incubated 10 min at 37°C with 0.5 ml/ml CFSE stock solution (5 mM in DMSO), and washed before injection. When indicated, the recipients were treated several times either with 200 μg per injection of anti-CTLA-4 mAb (clone UC10–4F10, PharMingen), with isotype-matched irrelevant hamster control antibody, or in some experiments, with PBS as a control. Mice were followed for diabetes daily, starting 7 days after transfer, for up to 3 weeks, testing for urine glucose levels and confirming positives by blood glucose measurements (41). Hematoxylin/eosin staining of thin sections from Bouin's solution-fixed, paraffin-embedded pancreata was performed as described (47). Multiple sections from each animal were scored for insulitis, at least 40 islets per individual. The analysis of tissue infiltration in CTLA-4o/o/NOD mice also was performed on hematoxylin-eosin-stained paraffin sections.
Antibodies and Flow Cytometry.
T cell analysis was performed as described (29, 40). The following mAbs were used: phycoerythrin-conjugated anti-CD4 (Caltag, South San Francisco, CA); allophycocyanin-conjugated anti-CD8 (Caltag); FITC-conjugated anti-CD69 (PharMingen); biotin-conjugated anti-CD25 (PharMingen); phycoerythrin-conjugated anti-IFN-γ (PharMingen); phycoerythrin-conjugated anti-IL-4 (PharMingen); FITC-conjugated anti-CD4 (Caltag); IM7, specific for CD44; and Mel-14, specific for CD62L (for references, see ref. 29). Biotinylated mAb was revealed by FITC-conjugated streptavidin and the other mAbs by FITC-conjugated goat anti-rat IgG (Jackson ImmunoResearch).
For intracellular staining, single-cell suspensions from lymph nodes were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate plus 500 ng/ml ionomycin for 4 h at 37°C in the presence of 10 mg/ml Brefeldin A. After fixation in 2% formaldehyde, the cells were stained intracellularly for cytokines in the presence of 0.3% saponin for cell permeabilization, followed by staining of the surface markers CD4 and CD8.
Results
Anti-CTLA-4 Does Not Exert Its Effect During the Priming of BDC2.5 T Cells in the PLNs.
As a first step, we established that the diabetogenic effect of anti-CTLA-4 mAb treatment, originally observed in juvenile BDC2.5 TCR tg mice (29), was recapitulated in the naive BDC2.5 T cell transfer system (40). Splenocytes from 10- to 12-day-old BDC2.5/NOD mice were transferred into adult T cell-deficient Cαo/o/NOD recipients that were or were not treated with anti-CTLA-4, and the fate of these cells and the effects they provoked were investigated. Such young BDC2.5/NOD animals were chosen as T cell donors because there are no signs of T cell activation in the PLNs at this age, and insulitis is not detectable until several days later (40). Therefore, the donor BDC2.5 T cells have not yet contacted their antigen, which resides specifically in the islets. When treated with anti-CTLA-4 mAb at the time of transfer, recipients consistently developed diabetes 9–12 days later; in contrast, control recipients only rarely showed disease (Table 1). Both types of recipients developed insulitis, but the lesions were strikingly different in the two cases when examined 7 days after transfer. Anti-CTLA-4 treatment induced a much more aggressive lesion, with generalized inflammation and destruction, instead of the mild and nondestructive lesions seen on sections from most control animals. Thus, the effects of anti-CTLA-4 treatment in the naive BDC2.5 T cell transfer system seem to mimic nicely the diabetogenic influence previously documented with juvenile BDC 2.5/NOD mice.
Table 1.
Diabetogenic effect of anti-CTLA-4 mAb treatment in the naive BDC2.5 T cell transfer system
| Treatment | Insulitis at d7 | Diabetes |
|---|---|---|
| Ctl mAb | Mild, nonaggressive | 3 /18 |
| α-CTLA-4 mAb | Aggressive | 7 /8 |
Adult Cαo/o/NOD hosts received 1–2 × 107 erythrocyte-depleted splenocytes from 10- to 12-day-old BDC2.5/NOD donors. The indicated mAbs were routinely given at the time of transfer and 3 days later.
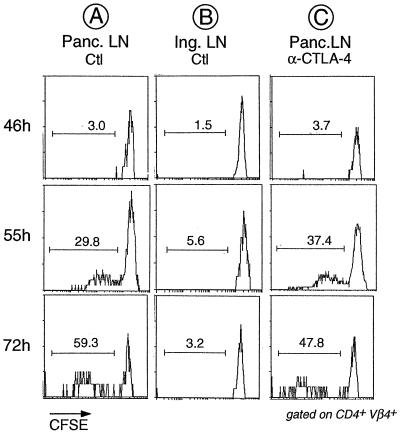
We then determined whether the effect of anti-CTLA-4 mAb treatment is already apparent during the initial priming phase, when BDC2.5 T cells first contact their antigen. In the transfer system, initial antigen encounter is readily detectable as a proliferation response restricted to the PLNs, measurable by using CFSE-labeled donor cells whose cycling can be traced by progressive dilution of the label with each cell division (40, 46). Hence, we tested whether anti-CTLA-4 treatment modified the kinetics or amplitude of this proliferation response. As illustrated in Fig. 1A, and in keeping with our previous observations (40), antigen-driven cell division was not detected at 46 h after transfer, but began after 50 h and progressed thereafter. Not all transgenic T cells proliferated during this time, as indicated by the preponderant population of undivided cells still present after 72 h. Proliferation was antigen specific because it was absent from lymph nodes other than the PLNs (Fig. 1B). Administration of anti-CTLA-4 at the time of transfer did not alter the proliferative behavior of the transferred BDC2.5 T cells. According to the data from multiple experiments, neither the kinetics of division nor the proportion of dividing cells were repeatably changed (e.g., Fig. 1C).
Figure 1.
No detectable effect of anti-CTLA-4 treatment on division of transferred BDC2.5 T cells in the PLNs. CFSE-labeled splenocytes from juvenile BDC2.5/NOD mice were transferred into adult Cαo/o/NOD animals that had been treated with anti-CTLA-4 mAb or PBS 2 h before transfer. PLNs and inguinal lymph node were removed from the recipients at the indicated times after transfer, and cells were stained with mAbs against CD4 and Vβ4 (the trangene-encoded TCR β-chain). Shown are histograms of CFSE staining for gated CD4+Vβ4+ cells. The reduction in CFSE staining intensity signifies that cell proliferation has taken place, and the proportion of divided cells is indicated.
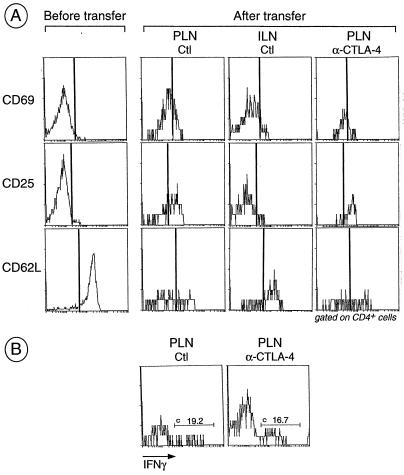
The effect of anti-CTLA-4 mAb treatment on cell activation also was investigated by analyzing the expression of activation markers 3 days after transfer. In PLNs of untreated recipients, up-regulation of the early activation markers CD69 and CD25, and down-regulation of CD62L was observed (Fig. 2A). In the control inguinal lymph nodes from the same recipient, these modulations of activation markers were markedly less pronounced, consistent with the fact that there was no antigen-specific proliferation in the inguinal lymph nodes (Fig. 1). Again, we could not detect any significant difference between anti-CTLA-4-injected and control mice. Lastly, according to results from intracellular staining experiments, levels of IFN-γ produced by PLN T cells were not distinguishable in the two types of recipients (Fig. 2B).
Figure 2.
Activation of BDC2.5 T cells after transfer into anti-CTLA-4-treated and control recipients. (A) Splenocytes from juvenile BDC2.5/NOD mice were pooled and an aliquot was stained for CD4, CD8, and early (CD25 and CD69) and late (CD62L) activation markers. Cells were transferred into adult Cαo/o/NOD mice that were treated with anti-CTLA-4 as described in the legend to Fig. 1. At day 3, single-cell suspensions from PLNs and inguinal lymph nodes were stained for CD4, CD8, and various activation markers; histograms gated on CD4+ cells are shown. (B) Same as in A except intracellular staining for IFN-γ was performed in place of staining for activation markers.
By these diverse criteria then, anti-CTLA-4 mAb treatment does not detectably influence the initial priming of BDC T cells in the PLNs.
Anti-CTLA-4 Acts When BDC2.5 T Cells Reencounter Their Antigen in the Target Organ, the Pancreatic Islets.
Although anti-CTLA-4 mAb treatment did not appear to affect BDC2.5 T cells during the priming phase, it was still possible that the homing of primed cells to the pancreatic islets was altered. We assayed this parameter by studying pancreas sections from recipients treated with anti-CTLA-4 or control mAb (Table 2). In both types of recipient, insulitis was still rare at 5 days after transfer, with very few infiltrated islets, in accordance with our previous data (40). However, the phenotype of the lesion was different in the two cases. In anti-CTLA-4-treated, but not control mAb-treated, mice, insulitis was immediately aggressive, also spreading into the surrounding exocrine tissue even when only one or two islets in the examined section were affected. This aggressive aspect was very reminiscent of islets from young BDC2.5 mice treated with anti-CTLA-4, as described (29). Insulitis had progressed rapidly by day 7; at this time point, control mice displayed the large “innocuous” infiltration typical of standard BDC2.5/NOD mice, whereas the infiltrate in anti-CTLA-4-treated animals persisted in its aggression (Table 2).
Table 2.
Comparison of insulitis development in animals administered α-CTLA-4 versus control mAb
| Treatment | Assessment of insulitis at day | No. of mice | % insulitis in individual mice |
|---|---|---|---|
| α-CTLA-4 | 5 | 6 | 9, 0, 12*, 3, 9*, 62* |
| Control | 5 | 7 | 0, 5, 9, 0, 0, 7, 2 |
| α-CTLA-4 | 7 | 2 | 94*, 79* |
| Control | 7 | 2 | 50, 5 |
Aggressive insulitis.
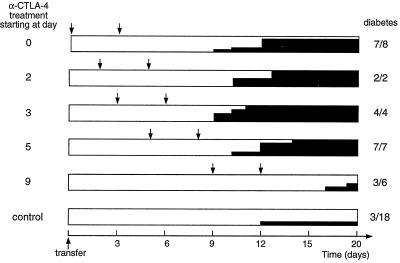
The results presented so far suggest that anti-CTLA-4 treatment does not have its effect until primed BDC2.5 T cells reach their target organ. This hypothesis predicts that anti-CTLA-4 would still be effective if administration were to be delayed. Thus, we injected mice that had received naive BDC2.5 T cells with anti-CTLA-4 at different times after transfer and monitored diabetes development (Fig. 3). Clearly, the mAb did not need to be present during the first days of the response to promote diabetes. Treatment starting at day 5 provoked disease as efficiently and rapidly as early administration. However, when the mAb treatment was delayed until day 9, a reduced fraction of the mice got diabetes (3 of 6) and disease started considerably later (between days 17 and 20).
Figure 3.
Diabetes development after treatment with anti-CTLA-4 mAb initiated at various times. Cαo/o/NOD recipients of BDC2.5 splenocytes were treated with anti-CTLA-4 mAb (2 × 200 μg) or control mAb or PBS starting on different days after transfer, as listed on the left. Arrows indicate the times of anti-CTLA-4 injection; solid black shading shows the incidence of diabetes; and the numbers on the right represent the final diabetes frequency. Inexplicably, in a few experiments, all mice became diabetic, whether they were treated with anti-CTLA-4 or not; data from these experiments were excluded from the results shown above.
This result needs to be considered in light of the very repeatable kinetics of the naive BDC2.5 T cell response as it unfolds in the transfer system (40): day 1/2–1, appearance in the PLNs; days 2–3, activation in the PLNs; and days 5–8, first signs of insulitis. Thus, the critical time window during which anti-CTLA-4 mAb exerts its diabetogenic effect appears to be subsequent to the priming phase, not until the activated BDC2.5 T cells have migrated to the pancreas and reencounter their antigen in the islets.
Results on CTLA-4-Deficient BDC2.5 Mice Bolster the Interpretation That Anti-CTLA-4 Operates by Blocking CTLA-4 Engagement.
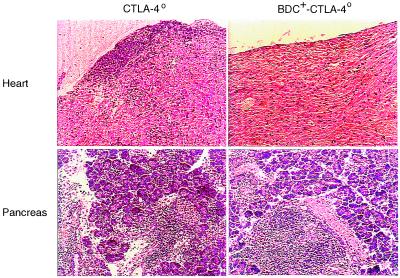
There can be important caveats to the interpretation of in vivo mAb-blocking experiments, and certain of these may be particularly applicable to studies with anti-CTLA-4. First, it is not always clear that the mAbs are merely blocking function and are not, instead, signaling through the target molecule, or worse, are inducing lysis of the cells expressing the marker. Second, there is always the possibility of mAb cross-reactivity with other molecules, above all when the target is a member of a continuously expanding multigene family, as CTLA-4 is (48). Therefore, we felt it important to confirm our major results on animals in which the CTLA-4 gene had been inactivated rather than its product putatively blocked. CTLA-4o/+ mice on the C57/BL6 background were crossed to BDC2.5/NOD mice for three generations before intercrossing to obtain CTLA-4o/o BDC2.5/NOD mice; in the process, animals were selected for homozygosity for H-2g7 at the MHC. CTLA-4-deficient mice usually die around 3 weeks of age because of massive lymphocyte proliferation and infiltration into multiple organs (30–32). This was also the case on the NOD genetic background in the absence of the BDC2.5 transgene: heart, liver, and pancreas from 3-week-old BDC2.5-negative CTLA-4o/o mice displayed extensive infiltration (Fig. 4), and these animals all died at 3–4 weeks of age. In contrast, littermates carrying the BDC2.5 transgenes survived beyond this point. Organs from 6-week-old mice were investigated, and no obvious infiltration was found except in the pancreas, which was heavily invaded (Fig. 4). We did note, however, that early (CD69 and CD25) and late (CD44) activation markers were up-regulated in lymphocytes from BDC2.5-positive CTLA-4o/o mice, which also had a slightly enlarged spleen [containing about 1.5 times the number of lymphocytes found in littermates expressing CTLA-4 (data not shown)].
Figure 4.
Organ infiltration in CTLA-4-deficient mice in the presence or absence of the BDC2.5 TCR transgenes. Organs were removed from 3-week-old CTLA-4o/o/NOD mice and 6-week-old BDC2.5-positive CTLA-4o/o/NOD mice, and paraffin sections were stained with hematoxylin/eosin. Only heart and pancreas sections are shown. The pictures are representative of three mice of each type.
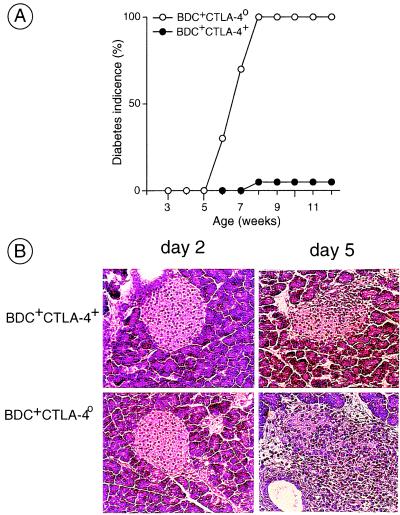
Reflecting the heavy insulitis, all BDC2.5-positive CTLA-4o/o mice became diabetic at an early age, between 5 and 7 weeks of age (Fig. 5A). In contrast, their BDC2.5-positive CTLA-4+/o littermates showed late and relatively rare diabetes, as is customary for this transgene on the NOD background (43).
Figure 5.
Diabetes promotion by CTLA-4-deficient BDC2.5 T cells. (A) CTLA-4o/+ mice were crossed three times to BDC2.5/NOD mice and intercrossed to obtain BDC2.5-positive CTLA-4o/o/NOD animals (selected for H-2g7 homozygosity). These offspring were followed for diabetes. (B) Splenocytes from BDC2.5-positive CTLA-4o/o/NOD mice and CTLA-4+ control littermates were transferred into Cαo/o/NOD recipients. Pancreata from individual recipients were removed at days 2 or 5 after transfer, and paraffin sections stained with hematoxylin/eosin were examined by histology.
Therefore, the BDC2.5 TCR transgenes were able to “cure” the CTLA-4o/o phenotype of generalized tissue infiltration, as did several other TCR transgenes (12, 35, 36, 49, 50), but the absence of CTLA-4 resulted in aggressive autoimmunity and rapid diabetes development in all mice—essentially the same phenotype as seen after the anti-CTLA-4 treatment of juvenile BDC2.5/NOD animals (29), although perhaps slightly slower.
We then used splenocytes from the CTLA-4-deficient BDC2.5 mice in transfer experiments. By 3 weeks after transfer into Cα0/0/NOD animals, most of the recipients of BDC2.5-positive CTLA-40/0 cells had developed diabetes, whereas recipients of littermate BDC2.5-positive CTLA-40/+ cells almost never did (data not shown). One difference between the two donor populations was that many of the former were in an activated state (see above), whereas the latter were essentially all naive. Therefore, we checked for immediate entry into the islets. The pancreata were removed and processed 2 and 5 days after transfer into recipients injected with splenocytes from BDC2.5-positive CTLA-4o/o mice or control littermates. At 2 days, none of the recipients showed any signs of insulitis (Fig. 5B Left); at day 5, insulitis began to appear in both types of recipient, but was much more aggressive in those transferred with BDC2.5-positive CTLA-4o/o T cells (Fig. 5B Right).
Thus, absence of a functional CTLA-4 gene provokes the same phenotype as anti-CTLA-4 mAb treatment, both in juvenile BDC2.5 mice at the onset of autoimmunity and in naive BDC2.5 T cell transfer experiments, supporting the notion that anti-CTLA-4 treatment in vivo does have a blocking function and that CTLA-4 is the effective target.
Discussion
By using the standard BDC2.5 TCR tg model of autoimmune diabetes, we previously established that CTLA-4 plays a pivotal role in determining the outcome of autoimmune aggression (29). We have now exploited a powerful BDC2.5-derived transfer system to demonstrate that CTLA-4 does not exert its effect when naive self-reactive T cells first contact their antigen during lymph node priming, but rather when activated T cells migrate to the target tissue and encounter antigen for a second time. Three lines of evidence were provided in support of this conclusion: (i) anti-CTLA-4 mAb treatment had no obvious effect on the early events of BDC2.5 T cell activation in the PLNs; (ii) anti-CTLA-4 did not need to be administered until 5 days after T cell transfer, the time at which BDC2.5 cells first invade the islets; and (iii) CTLA-4-deficient BDC2.5 T cells also exhibited altered, decidedly more aggressive behavior on entry into the islets 5 days after transfer.
This conclusion is consistent with several published findings on CTLA-4. First, CTLA-4 does not appear in any abundance at the cell surface until 2–3 days after activation of primary T cells, hinting that it might not have a major function until then (7). Second, the effect of the CTLA-4-null mutation was much more pronounced on secondary than on primary responses of antigen-specific CD8+ T cells (12). Whereas significant effects on both primary and secondary antigen-specific CD4+ T cell responses have been documented, the latter were typically more profound (35, 36). Third, Shrikant et al. (37) demonstrated that CTLA-4 engagement can prevent the continuation of a response to a transfected tumor antigen in the absence of an effect on the initial response. Fourth, Issazadeh et al. (39) have described a system of acquired thymic tolerance to myelin basic protein antigens that depends on CTLA-4 engagement during a narrow time window 3 days after antigen priming. Finally, our conclusion is consistent with the basic phenotype of CTLA-4-deficient mice, the dominant feature being tissue infiltration rather than the isolated lymphoproliferation of lpr mice (30–32).
Why the retarded CTLA-4 function? One possibility is that the delay merely reflects the expression patterns of costimulatory receptors on the T cells and/or costimulatory ligands on the antigen-presenting cells. Concerning the former, it may be that CTLA-4 does not appear on the cell surface at a sufficiently high level until the T cells have arrived in the target tissue. Concerning the latter, it may be that the amounts or forms of B7–1 and B7–2 differ in the target tissue and the draining lymph nodes. However, we have not so far noticed any differences in expression of B7 molecules on dendritic cells residing in the pancreatic islets and PLNs of juvenile NOD mice (data not shown). A second possibility is that more antigen is available in the pancreas than in the PLNs, and that CTLA-4/B7 interactions would operate only at the higher antigen concentrations. This would be consistent with the ability of antigen-presenting cells isolated from the former but not the latter site to stimulate BDC2.5 T cells in an in vitro assay (51). Third, it is possible that the relevant negative signaling pathways downstream of CTLA-4 engagement are properly hooked up only after T cell activation, a scenario that also might explain some of the early confusion regarding the inhibitory versus activatory role of CTLA-4 (52). If this turns out to be true, the delayed influence of CTLA-4 documented in this and other in vivo situations will need to be reconciled with some early biochemical effects reported in in vitro experiments, including effects immediately downstream of the TCR in activatory signaling pathways and on several transcription factors activated early by TCR/CD28 signaling (9, 10, 13, 53, 54). A fourth possibility, suggested from very recent studies on mouse models of experimental allergic encephalomyelitis and diabetes (55), is that CTLA-4 is not actually expressed in the BDC2.5 effector cells, but rather on regulatory cells, perhaps not encountered until invasion of the islets.
Our earlier results (29) demonstrated that anti-CTLA-4 treatment had no effect when administered after insulitis was already entrenched in BDC2.5/NOD mice. Viewed together, these and the present findings indicate that CTLA-4 controls the “temper” of activated lymphocytes when they first invade the islets, but only within a restricted time window. Once established, the innocuous form of insulitis is stable in the absence of CTLA-4, perhaps depending on other members of the costimulatory receptor family, such as ICOS (48), for maintenance.
BDC2.5 TCR tg mice carrying the CTLA-4 knockout mutation had disease characteristics highly reminiscent of anti-CTLA-4 treated animals. Whereas both types of experiments have potential caveats (the complications of linked loci for the former, complexities discussed above for the latter), the convergence of results should assuage lingering doubts about the blocking versus enhancing effects of the UC10–4F10 mAb. However, CTLA-4-deficient BDC2.5 TCR tg mice did develop diabetes a bit later than mAb-treated mice (5–7 weeks vs. 21 days of age). One explanation could be that in CTLA-4-deficient mice, the negative signal is absent from inception, prompting other negative costimulatory molecules to partially compensate for the function of CTLA-4 in an adaptive process. This would not be sufficient to prevent the phenotype of early-onset diabetes but could result in a delay. On the other hand, if CTLA-4 is suddenly blocked by mAb treatment, there would not be the opportunity for such adaptations to take place.
In summary, the conclusion from our diverse analyses points to a very particular role for CTLA-4 engagement in controlling autoaggression. It does not dampen initial responses in the lymphoid organs, but controls the outcome of secondary recognition of antigens in tissues, consistent with an important role in peripheral tolerance to tissue-specific antigens. It should prove further revealing to identify the antigen-presenting cell and ligand that mediate the negative signals involved.
Acknowledgments
We thank C. Waltzinger for help with the flow cytometry, T. Ding for doing the pancreas sections, F. Fischer and V. Louerat for maintaining the mice, W. Magnant for help in diabetes testing, and J. Hergueux, P. Gerber, and C. Ebel for technical assistance. This work was supported by grants to C.B. and D.M. from the European Union, and institute funds from the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, and the Centre Hospitalier Universitaire Régional. F.L. received a fellowship from the Deutsche Forschungsgemeinschaft (lu 634/1–1) and the European Union.
Abbreviations
- CFSE
5,6-carboxfluorescein diacetate succinimidyl ester
- PLN
pancreatic lymph node
- TCR
T cell receptor
- tg
transgenic
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200348397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200348397
References
- 1.Oosterwegel M A, Greenwald R J, Mandelbrot D A, Lorsbach R B, Sharpe A H. Curr Opin Immunol. 1999;11:294–300. doi: 10.1016/s0952-7915(99)80047-8. [DOI] [PubMed] [Google Scholar]
- 2.Chambers C A, Allison J P. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 3.McAdam A J, Schweitzer A N, Sharpe A H. Immunol Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 4.Linsley P S, Greene J L, Tan P, Bradshaw J, Ledbetter J A, Anasetti C, Damle N K. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Guo Y, Huang A, Zheng P, Liu Y. J Exp Med. 1997;185:1327–1335. doi: 10.1084/jem.185.7.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng P, Wu Y, Guo Y, Lee C, Liu Y. Proc Natl Acad Sci USA. 1998;95:6284–6289. doi: 10.1073/pnas.95.11.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walunas T L, Lenschow D J, Bakker C Y, Linsley P S, Freeman G J, Green J M, Thompson C B, Bluestone J A. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 8.Krummel M F, Allison J P. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krummel M F, Allison J P. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walunas T L, Bakker C Y, Bluestone J A. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alegre M-L, Shiels H, Thompson C B, Gajewski T F. J Immunol. 1998;161:3347–3356. [PubMed] [Google Scholar]
- 12.Chambers C A, Sullivan T J, Truong T, Allison J P. Eur J Immunol. 1998;28:3137–3143. doi: 10.1002/(SICI)1521-4141(199810)28:10<3137::AID-IMMU3137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Brunner M C, Chambers C A, Chan F K-M, Hanke J, Winoto A, Allison J P. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 14.Chen W, Jin W, Wahl S M. J Exp Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallarino F, Fields P E, Gajewski T F. J Exp Med. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCoy K D, Hermans I F, Fraser J H, Le Gros G, Ronchese F. J Exp Med. 1999;189:1157–1162. doi: 10.1084/jem.189.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney E R, Walunas T L, Karr R W, Morton P A, Loh D Y, Bluestone J A, Jenkins M K. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 18.Krummel M F, Sullivan T J, Allison J P. Int Immunol. 1996;8:519–523. doi: 10.1093/intimm/8.4.519. [DOI] [PubMed] [Google Scholar]
- 19.Blazar B R, Taylor P A, Panoskaltsis-Mortari A, Sharpe A H, Vallera D A. J Immunol. 1999;162:6368–6377. [PubMed] [Google Scholar]
- 20.Lin H L, Rathmell J C, Gray G S, Thompson C G, Leiden J M, Alegre M-L. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judge T A, Wu Z, Zheng X-G, Sharpe A H, Sayegh M H, Turka L A. J Immunol. 1999;162:1947–1951. [PubMed] [Google Scholar]
- 22.McCoy K, Camberis M, Le Gros G. J Exp Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walunas T L, Bluestone J A. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]
- 24.Leach D R, Krummel M F, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 25.Kwon E D, Hurwitz A A, Foster B A, Madias C, Feldhaus A L, Greenberg N M, Burg M B, Allison J P. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurwitz A A, Yu T F Y, Leach D R, Allison J P. Proc Natl Acad Sci USA. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrin P J, Maldonado J H, Davis T A, June C H, Racke M K. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 28.Karandikar N J, Vanderlugt C L, Walunas T L, Miller S D, Bluestone J A. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lühder F, Höglund P, Allison J P, Benoist C, Mathis D. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Science. 1995;270:985–989. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 31.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Sharpe A H. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 32.Chambers C A, Cado D, Truong T, Allison J P. Proc Natl Acad Sci USA. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metz D P, Farber D L, Taylor T, Bottomly K. J Immunol. 1998;161:5855–5861. [PubMed] [Google Scholar]
- 34.Scheipers P, Reiser H. Proc Natl Acad Sci USA. 1998;95:10083–10088. doi: 10.1073/pnas.95.17.10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oosterwegel M A, Mandelbrot D A, Boyd S D, Lorsbach R B, Jarrett D Y, Abbas A K, Sharpe A H. J Immunol. 1999;163:2634–2639. [PubMed] [Google Scholar]
- 36.Chambers C A, Kuhns M S, Allison J P. Proc Natl Acad Sci USA. 1999;96:8603–8608. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shrikant P, Khoruts A, Mescher M F. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 38.Perez V L, Parijs L V, Biuckians A, Zheng X X, Strom T B, Abbas A K. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 39.Issazadeh S, Zhang M, Sayegh M H, Khoury S J. J Immunol. 1999;162:761–765. [PubMed] [Google Scholar]
- 40.Höglund P, Mintern J, Heath W, Benoist C, Mathis D. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz J D, Wang B, Haskins K, Benoist C, Mathis D. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 42.Haskins K, McDuffie M. Science. 1990;249:1433–1436. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez A, Katz J D, Mattei M G, Kikutani H, Benoist C, Mathis D. Immunity. 1997;7:873–883. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- 44.Philpott K L, Viney J L, Kay G, Rastan S, Gardiner E M, Chae S, Hayday A C, Owen M J. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 45.Katz J D, Benoist C, Mathis D. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 46.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 47.Böhme J, Schuhbaur B, Kanagawa O, Benoist C, Mathis D. Science. 1990;249:293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- 48.Hutloff A, Dittrich A M, Beier K C, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek R A. Nature (London) 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 49.Waterhouse P, Backmann M F, Penninger J M, Ohashi P S, Mak T W. Eur J Immunol. 1997;27:1887–1892. doi: 10.1002/eji.1830270811. [DOI] [PubMed] [Google Scholar]
- 50.Bachmann M F, Waterhouse P, Speiser D E, McKall-Faienza K, Mak T W, Ohashi P S. J Immunol. 1998;160:95–100. [PubMed] [Google Scholar]
- 51.Green E A, Eynon E E, Flavell R A. Immunity. 1998;9:733–743. doi: 10.1016/s1074-7613(00)80670-6. [DOI] [PubMed] [Google Scholar]
- 52.Linsley P S. J Exp Med. 1995;182:289–292. doi: 10.1084/jem.182.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee K-M, Chuang E, Griffin M, Khattri R, Hong D K, Zhang W, Straus D, Samelson L E, Thompson C B, Bluestone J A. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 54.Olsson C, Riebeck K, Dohlsten M, Michaelsson E. J Biol Chem. 1999;274:14400–14405. doi: 10.1074/jbc.274.20.14400. [DOI] [PubMed] [Google Scholar]
- 55.Salomon B, Lenschow D J, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone J A. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]