Abstract
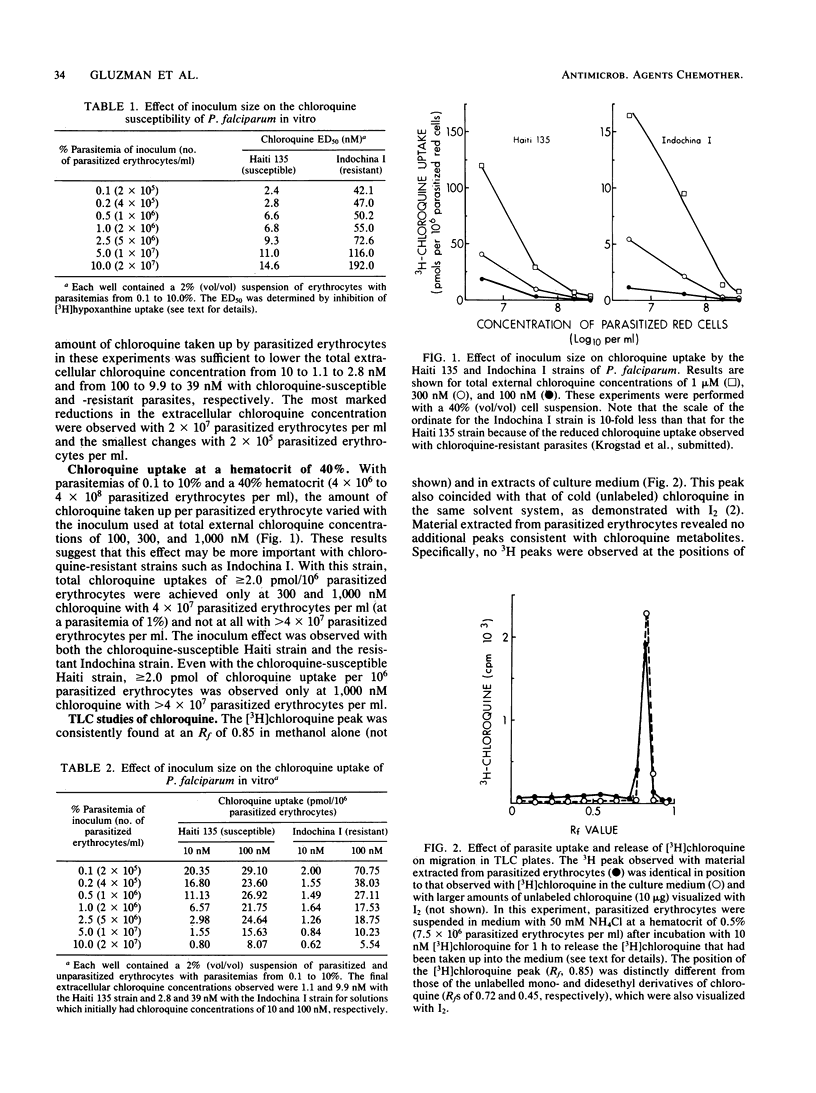
In the studies reported here, we examined the inoculum effect observed with chloroquine and Plasmodium falciparum. The 50% effective doses observed with both chloroquine-susceptible and -resistant parasites increased five- to sevenfold from their baseline values as the inoculum was increased from 2 X 10(5) to 2 X 10(7) parasitized erythrocytes per ml (parasitemias of 0.1 to 10% with a hematocrit of 2%). Increasing the inoculum also decreased the chloroquine uptake per parasitized erythrocyte 15- to 20-fold with both chloroquine-susceptible and -resistant parasites. However, because of the 100-fold increase in the inoculum, the total amount of chloroquine taken up actually increased sufficiently to reduce the extracellular chloroquine concentration in vitro by 60 to 90%. These studies suggest that a chloroquine uptake of greater than or equal to 2.0 pmol/10(6) parasitized erythrocytes is necessary for chloroquine to inhibit parasite growth. More marked reductions in the amount of chloroquine uptake per parasitized erythrocyte were observed with a hematocrit of 40% using similar parasitemias of 0.1 to 10% (inocula of 4 X 10(6) to 4 X 10(8) parasitized erythrocytes per ml). Thin-layer chromatography of [3H]chloroquine taken up by chloroquine-resistant P. falciparum revealed no evidence of drug alteration by the parasite. These studies define the mechanism responsible for the inoculum effect observed with chloroquine and P. falciparum in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelusi S. A., Dawodu A. H., Salako L. A. Kinetics of the uptake and elimination of chloroquine in children with malaria. Br J Clin Pharmacol. 1982 Oct;14(4):483–487. doi: 10.1111/j.1365-2125.1982.tb02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner R. W., Earle D. P., Taggart J. V., Zubrod C. G., Welch W. J., Conan N. J., Bauman E., Scudder S. T., Shannon J. A. STUDIES ON THE CHEMOTHERAPY OF THE HUMAN MALARIAS. VI. THE PHYSIOLOGICAL DISPOSITION, ANTIMALARIAL ACTIVITY, AND TOXICITY OF SEVERAL DERIVATIVES OF 4-AMINOQUINOLINE. J Clin Invest. 1948 May;27(3 Pt 2):98–107. doi: 10.1172/JCI101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranston H. A., Boylan C. W., Carroll G. L., Sutera S. P., Williamson J. R., Gluzman I. Y., Krogstad D. J. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science. 1984 Jan 27;223(4634):400–403. doi: 10.1126/science.6362007. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essien E. E., Afamefuna G. C. Chloroquine and its metabolites in human cord blood, neonatal blood, and urine after maternal medication. Clin Chem. 1982 May;28(5):1148–1152. [PubMed] [Google Scholar]
- Fitch C. D. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science. 1970 Jul 17;169(3942):289–290. doi: 10.1126/science.169.3942.289. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. L., Walker O., Alván G., Beermann B., Estevez F., Gleisner L., Lindström B., Sjöqvist F. Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol. 1983 Apr;15(4):471–479. doi: 10.1111/j.1365-2125.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. C., Krogstad D. J. Spectrophotometric assessment of dose-response curves for single antimicrobial agents and antimicrobial combinations. J Infect Dis. 1983 Apr;147(4):758–764. doi: 10.1093/infdis/147.4.758. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. A perspective on antimalarial action: effects of weak bases on Plasmodium falciparum. Biochem Pharmacol. 1986 Feb 15;35(4):547–552. doi: 10.1016/0006-2952(86)90345-x. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H., Gluzman I. Y. Antimalarials increase vesicle pH in Plasmodium falciparum. J Cell Biol. 1985 Dec;101(6):2302–2309. doi: 10.1083/jcb.101.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Nielsen R. L., Kohler R. B., Chin W., McCarthy L. J., Luft F. C. The use of exchange transfusions: a potentially useful adjunct in the treatment of fulminant falciparum malaria. Am J Med Sci. 1979 May-Jun;277(3):325–329. [PubMed] [Google Scholar]
- Pfaller M. A., Krogstad D. J. Imidazole and polyene activity against chloroquine-resistant Plasmodium falciparum. J Infect Dis. 1981 Oct;144(4):372–375. doi: 10.1093/infdis/144.4.372. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Krogstad D. J. Oxygen enhances the antimalarial activity of the imidazoles. Am J Trop Med Hyg. 1983 Jul;32(4):660–665. doi: 10.4269/ajtmh.1983.32.660. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Krogstad D. J., Parquette A. R., Nguyen-Dinh P. Plasmodium falciparum: stage-specific lactate production in synchronized cultures. Exp Parasitol. 1982 Dec;54(3):391–396. doi: 10.1016/0014-4894(82)90048-0. [DOI] [PubMed] [Google Scholar]
- Ponnudurai T., Leeuwenberg A. D., Meuwissen J. H. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop Geogr Med. 1981 Mar;33(1):50–54. [PubMed] [Google Scholar]
- Seaberg L. S., Parquette A. R., Gluzman I. Y., Phillips G. W., Jr, Brodasky T. F., Krogstad D. J. Clindamycin activity against chloroquine-resistant Plasmodium falciparum. J Infect Dis. 1984 Dec;150(6):904–911. doi: 10.1093/infdis/150.6.904. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Teklehaimanot A., Nguyen-Dinh P., Collins W. E., Barber A. M., Campbell C. C. Evaluation of sporontocidal compounds using Plasmodium falciparum gametocytes produced in vitro. Am J Trop Med Hyg. 1985 May;34(3):429–434. doi: 10.4269/ajtmh.1985.34.429. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]


