Abstract
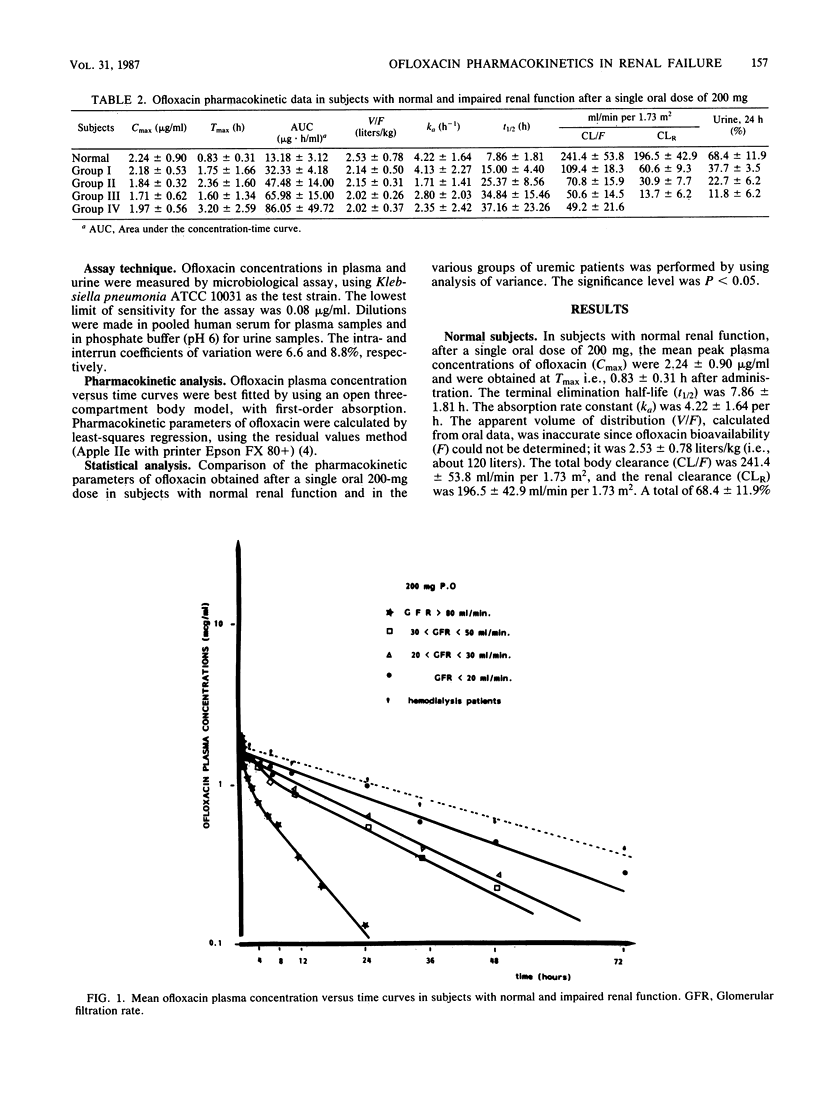
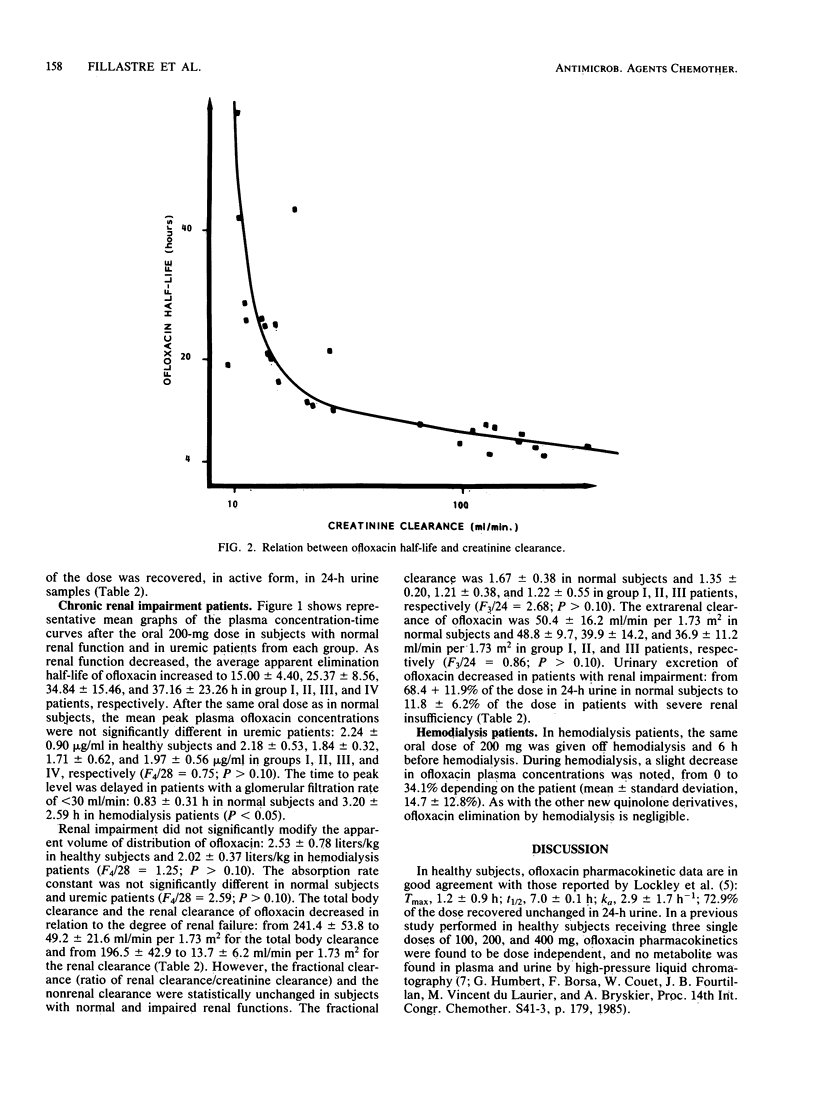
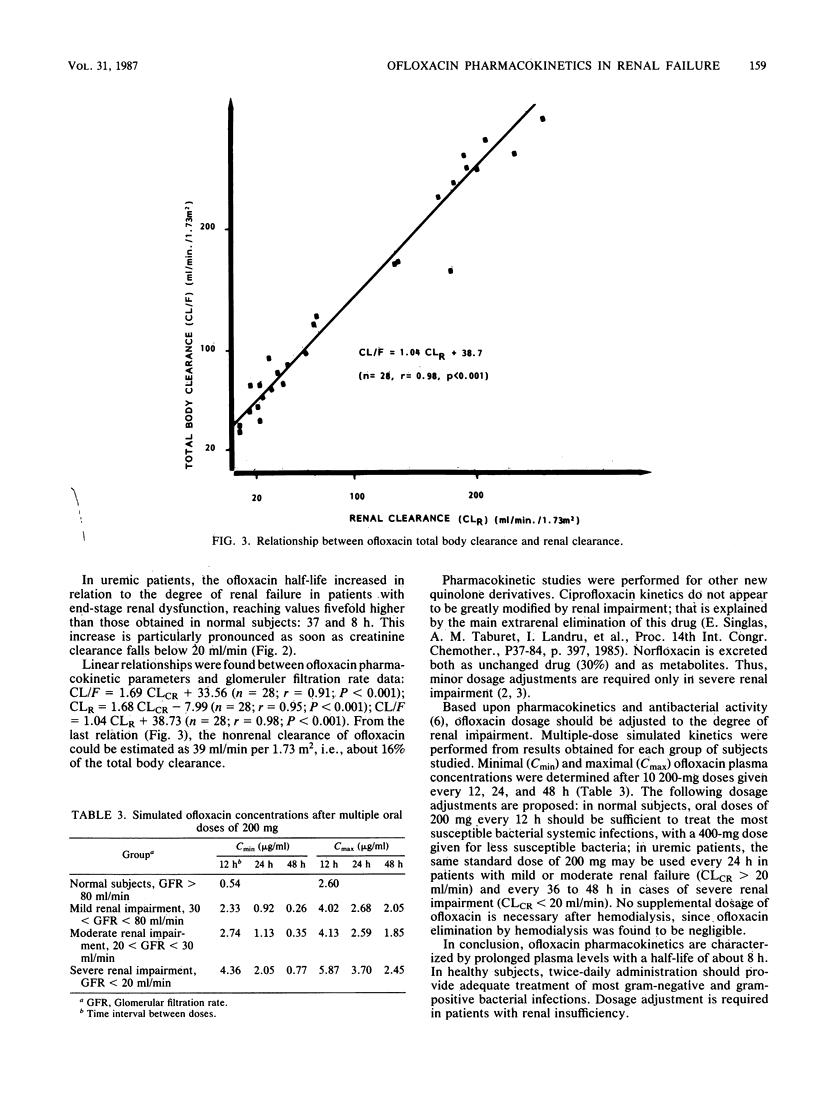
The pharmacokinetics of ofloxacin were investigated in 12 normal subjects and 21 uremic patients after the administration of a single oral 200-mg dose. An open three-compartment body model was used to calculate ofloxacin pharmacokinetic parameters. In healthy subjects, the peak plasma level averaged 2.24 +/- 0.90 micrograms/ml and was obtained at 0.83 +/- 0.31 h. The absorption rate constant was 4.22 +/- 1.64 h-1. The terminal half-life was 7.86 +/- 1.81 h. The apparent volume of distribution was 2.53 +/- 0.78 liters/kg. Total body and renal clearances were 241.4 +/- 53.8 and 196.5 +/- 42.9 ml/min per 1.73 m2, respectively. A total of 68.4 +/- 11.9% of the dose was recovered unchanged in 24-h urine. In uremic patients, the terminal half-life increased in relation to the degree of renal failure: from 8 h in normal subjects to 37 h in severely uremic patients. Renal insufficiency did not significantly modify the peak plasma level, the apparent volume of distribution, the fractional clearance, or the nonrenal clearance of ofloxacin. However, the time to peak level was delayed in patients with creatinine clearance of less than 30 ml/min. Linear relationships were found between ofloxacin pharmacokinetic parameters and glomerular filtration rate data. Ofloxacin is only very slightly removed by hemodialysis. Dosage adjustments of ofloxacin in uremic patients are proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eandi M., Viano I., Di Nola F., Leone L., Genazzani E. Pharmacokinetics of norfloxacin in healthy volunteers and patients with renal and hepatic damage. Eur J Clin Microbiol. 1983 Jun;2(3):253–259. doi: 10.1007/BF02029528. [DOI] [PubMed] [Google Scholar]
- Fillastre J. P., Hannedouche T., Leroy A., Humbert G. Pharmacokinetics of norfloxacin in renal failure. J Antimicrob Chemother. 1984 Oct;14(4):439–439. doi: 10.1093/jac/14.4.439. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley M. R., Wise R., Dent J. The pharmacokinetics and tissue penetration of ofloxacin. J Antimicrob Chemother. 1984 Dec;14(6):647–652. doi: 10.1093/jac/14.6.647. [DOI] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]


