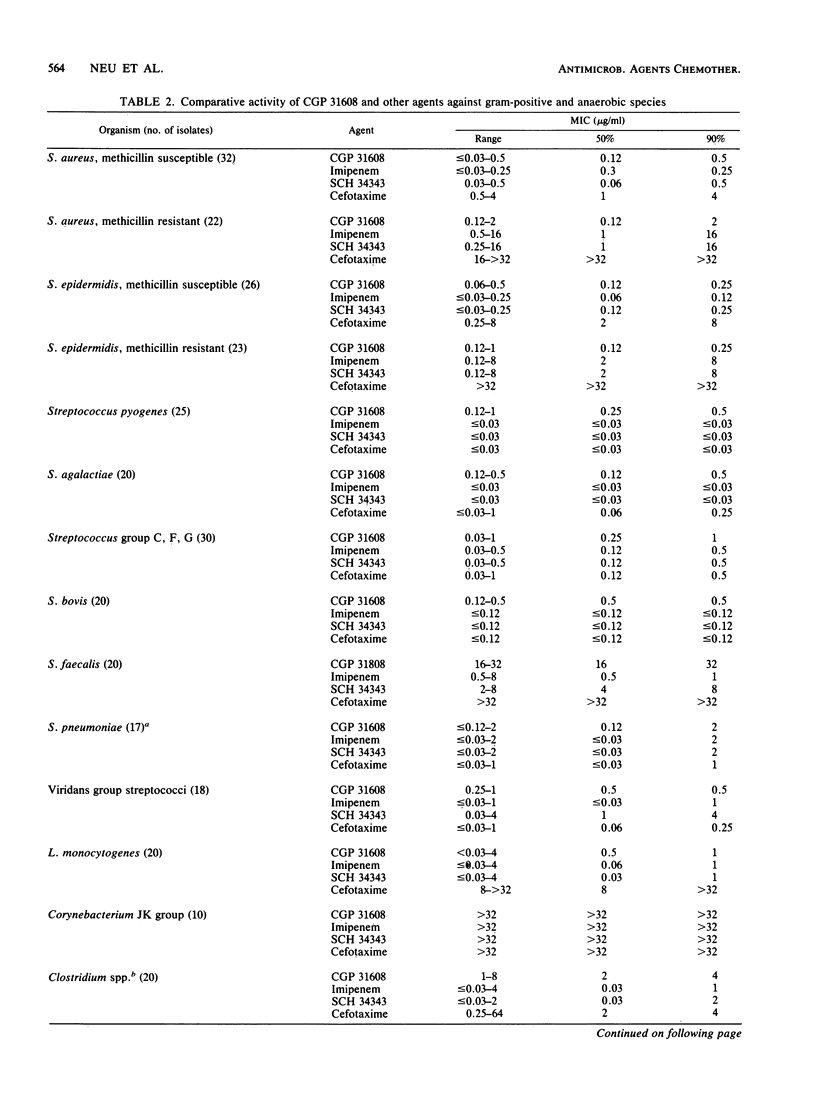
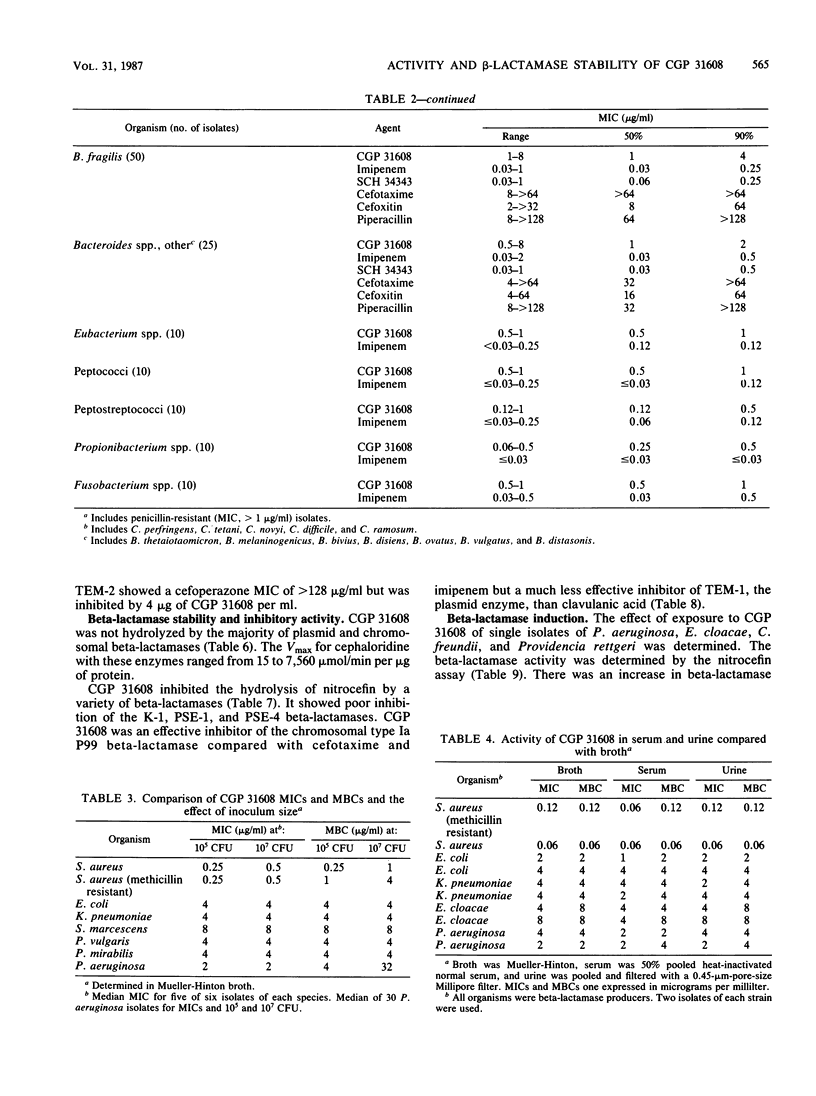
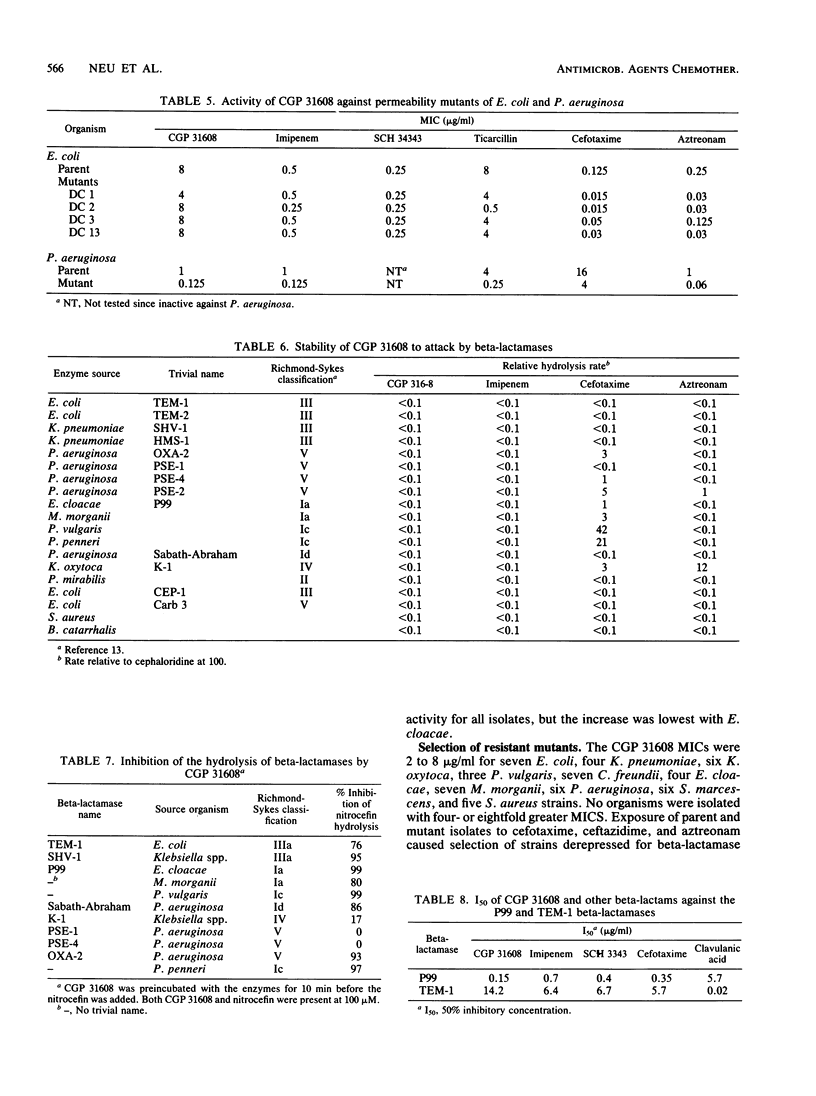
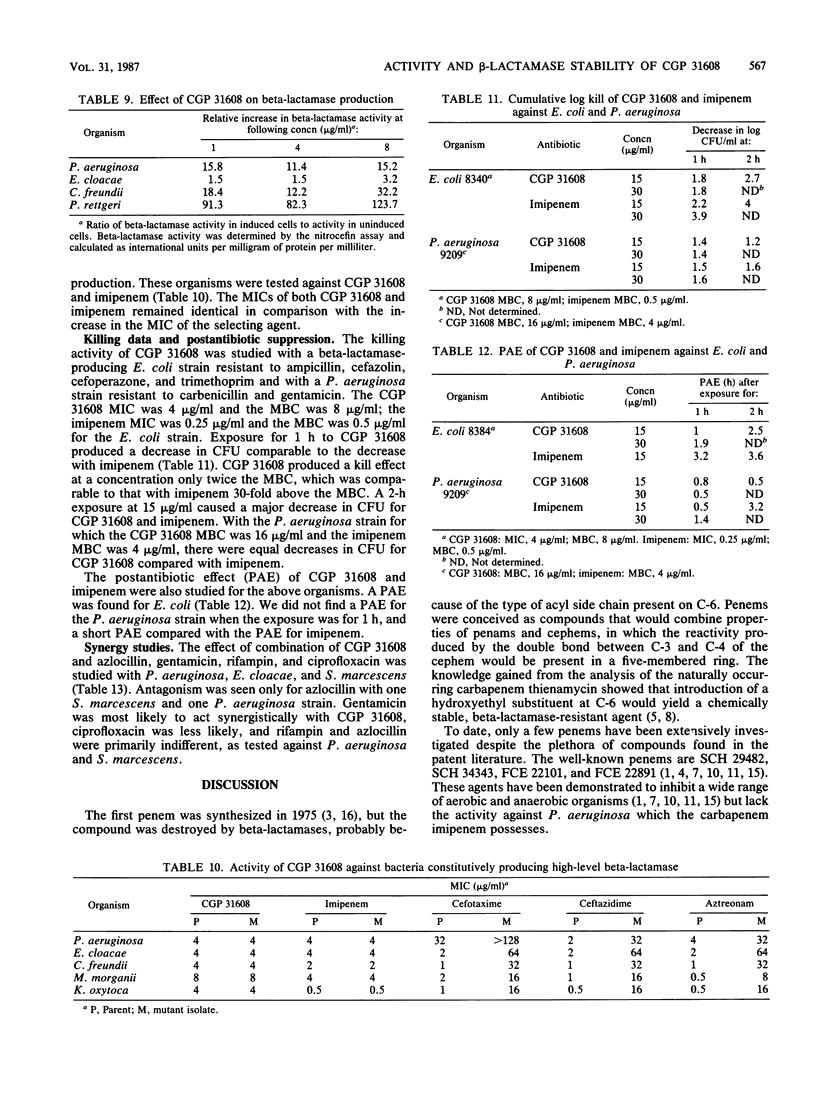
Abstract
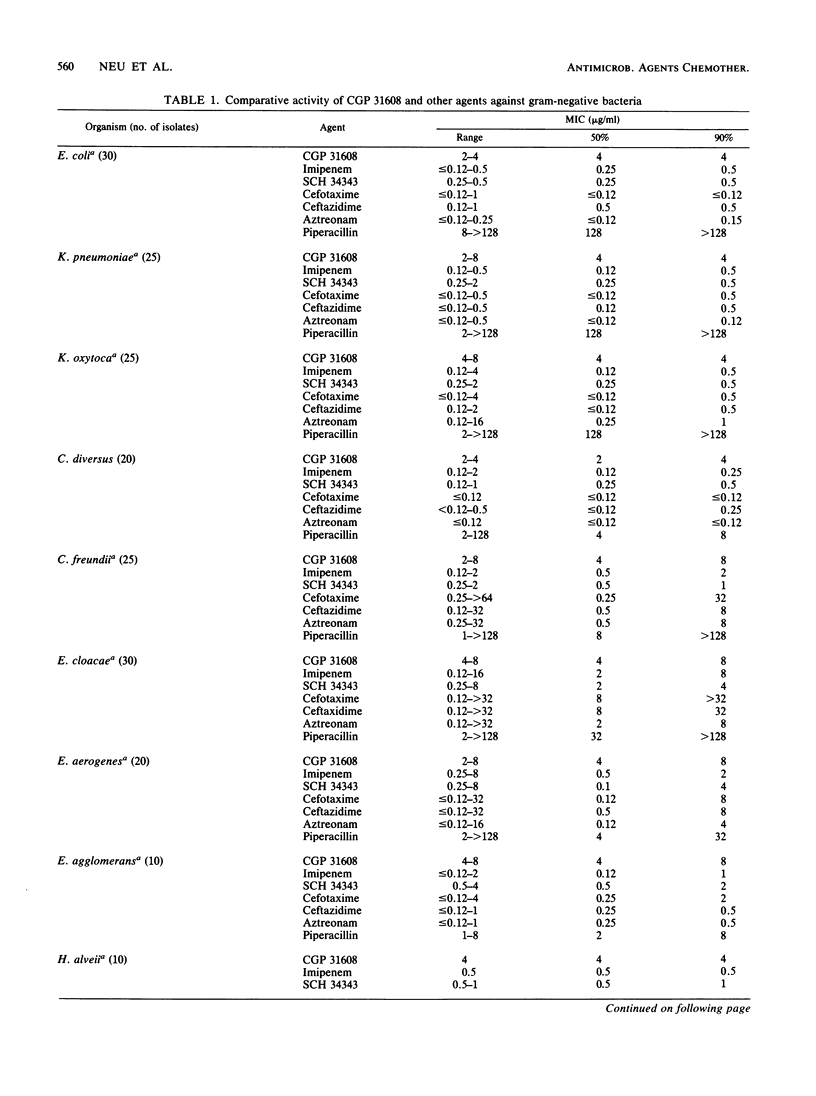
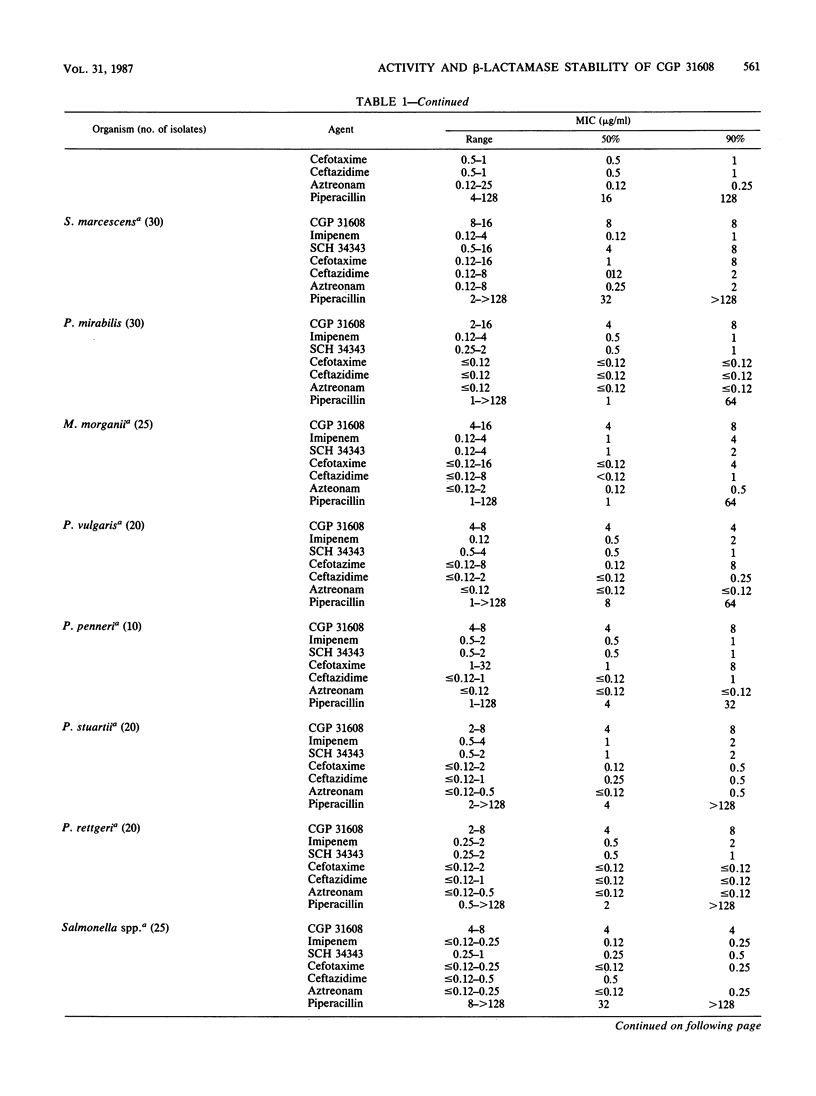
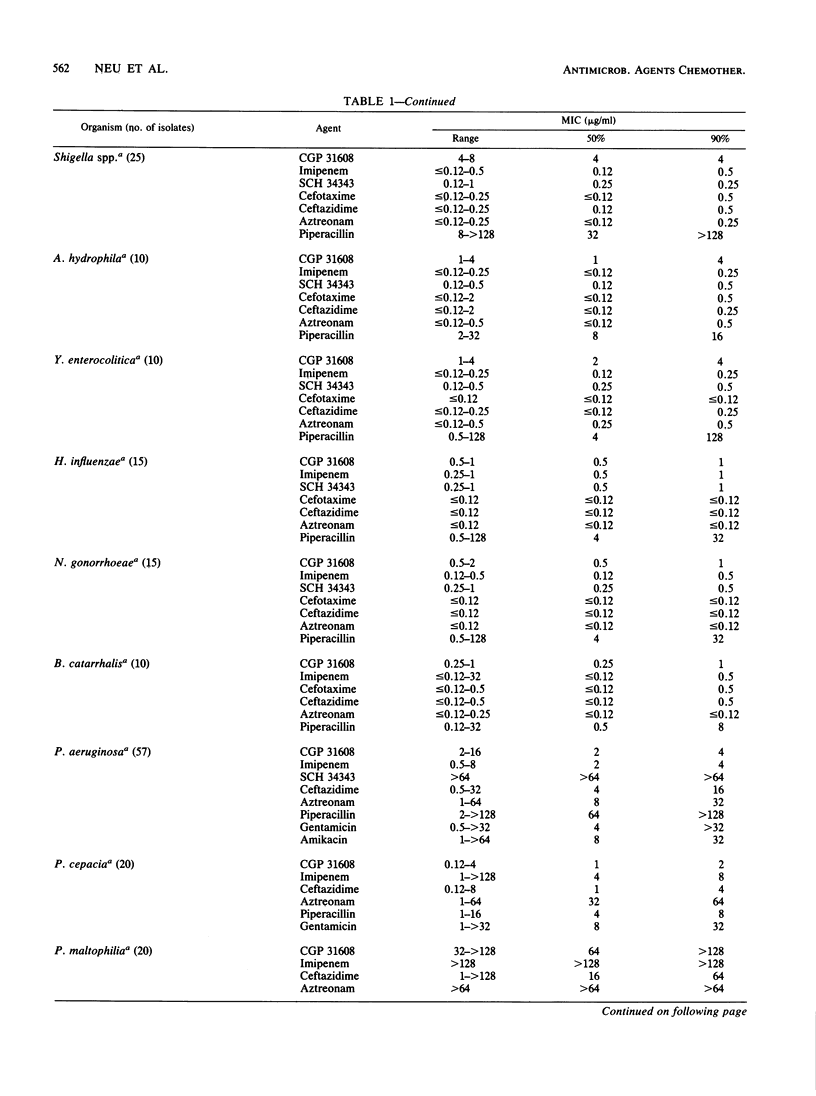
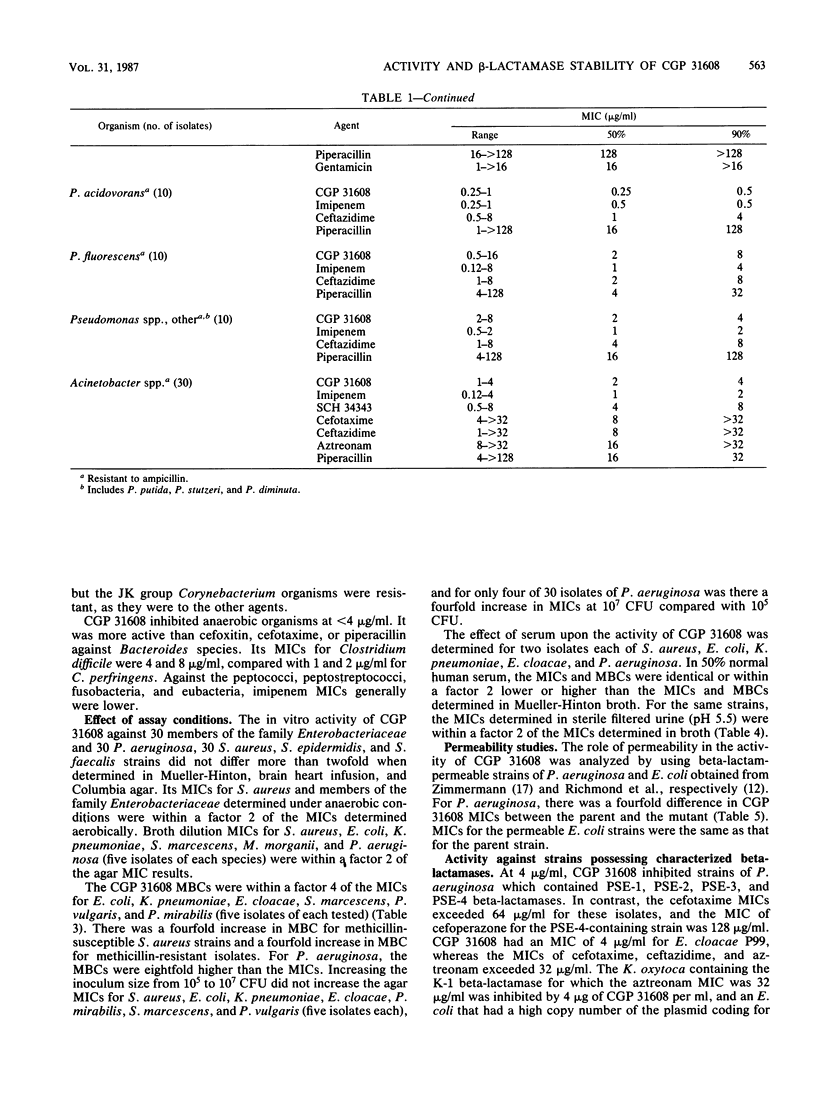
The in vitro activity of CGP 31608, a new penem, against aerobic and anaerobic organisms was evaluated and compared with those of other beta-lactams. CGP 31608 inhibited Escherichia coli, Klebsiella pneumoniae, K. oxytoca, Proteus mirabilis, Citrobacter diversus, and Salmonella, Shigella, Aeromonas, and Yersinia spp. with MICs for 50% of the strains (MIC50s) of 2 to 4 micrograms/ml and MIC90s of 4 micrograms/ml, compared with cefotaxime, ceftazidime, aztreonam, and imipenem MICs of less than 0.25 microgram/ml. MIC90s were 8 micrograms/ml for Enterobacter species and C. freundii, for which other agents had MICs of 32 micrograms/ml, except imipenem, which had equal activity. The MIC90 for Proteus vulgaris, Morganella morganii, Providencia stuartii, and Providencia rettgeri was 8 micrograms/ml, compared with less than 2 micrograms/ml shown by the other agents. Acinetobacter species resistant to other agents except imipenem were inhibited by 4 micrograms/ml, as were Pseudomonas aeruginosa, including piperacillin-, ceftazidime-, and gentamicin-resistant isolates. The MIC for P. cepacia, P. fluorescens, and P. acidovorans was less than or equal to 8 micrograms/ml, but that for P. maltophilia was greater than or equal to 128 micrograms/ml. Hemolytic streptococci A, B, C, G, and F were inhibited by less than 1 micrograms/ml, but the MIC for Streptococcus faecalis was greater than or equal to 32 micrograms/ml. MICs for Staphylococcus aureus methicillin-susceptible and -resistant strains were less than or equal to 1 microgram/ml, as were those for methicillin-susceptible and -resistant S. epidermidis. Bacteroides fragilis and Clostridium species and Fusobacterium spp. were inhibited by less than or equal to 4 micrograms/ml. CGP 31608 was not hydrolyzed by plasmid beta-lactamases TEM-1, TEM-2, SHV-1, PSE-1, OXA-2, PSE-4, or by S. aureus. Chromosomal beta-lactamases of type Ia in Enterobacter cloacae P99 and Morganella morganii, Ic in P. vulgaris, K-1 in K. oxytoca, and Id in P. aeruginosa also did not hydrolyze CGP 31608. It inhibited TEM-1, but the 50% inhibitory concentration was 14.2 micrograms/ml compared with 0.15 micrograms/ml for the P99 enzyme. CGP 31608 induced beta-lactamases in P. aeruginosa, E. cloacae, C. freundii and Providencia rettgeri, but there was no increase in MICs for the isolates and it did not select strains derepressed for beta-lactamase production. Synergy of CGP 31608 and gentamicin was found against 90% P. aeruginosa, 60% Enterobacter cloacae, and 50% Serratia marcescens strains. No synergy was found with rifampin. A postantibiotic effect was found against E. coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruna C. D., Jabes D., Sebben G., Sanfilippo A. In vitro and in vivo evaluation of the penem FCE 22101 and its orally absorbed ester FCE 22891. G Ital Chemioter. 1983 May-Dec;30(2-3):125–130. [PubMed] [Google Scholar]
- Ganguly A. K., Afonso A., Girijavallabhan V. M., McCombie S. Synthesis and preliminary in-vitro profile of Sch 34343--a new penem antibacterial agent. J Antimicrob Chemother. 1985 Jun;15 (Suppl 100):1–4. doi: 10.1093/jac/15.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Kropp H., Sundelof J. G., Birnbaum J. Thienamycin: development of imipenen-cilastatin. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):1–35. doi: 10.1093/jac/12.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Loebenberg D., Miller G. H., Moss E. L., Jr, Oden E., Hare R. S., Chung M., Waitz J. A. Evaluation of the in-vitro activity of Sch 29482. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):221–231. doi: 10.1093/jac/9.suppl_c.221. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Labthavikul P. In-vitro activity and beta-lactamase stability and inhibitory properties of a new penem antibiotic, Sch 34343. J Antimicrob Chemother. 1985 Jun;15 (Suppl 100):25–37. doi: 10.1093/jac/15.suppl_c.25. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Labthavikul P. The in-vitro activity of a novel penem FCE 22101 compared to other beta-lactam antibiotics. J Antimicrob Chemother. 1985 Sep;16(3):305–313. doi: 10.1093/jac/16.3.305. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Danks G. Comparison of in vitro activity of FCE 22101, a new penem, with those of other beta-lactam antibiotics. Antimicrob Agents Chemother. 1983 Dec;24(6):909–914. doi: 10.1128/aac.24.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration through the gram-negative cell wall: a co-determinant of the efficacy of beta-lactam antibiotics. Int J Clin Pharmacol Biopharm. 1979 Mar;17(3):131–134. [PubMed] [Google Scholar]


