Abstract
Neurons in the mammalian suprachiasmatic nuclei (SCN) generate daily rhythms in physiology and behavior, but it is unclear how they maintain and synchronize these rhythms in vivo. We hypothesized that parallel signaling pathways in the SCN are required to synchronize rhythms in these neurons for coherent output. We recorded firing and clock-gene expression patterns while blocking candidate signaling pathways for at least 8 days. GABAA and GABAB antagonism increased circadian peak firing rates and rhythm precision of cultured SCN neurons, but Gi/o did not impair synchrony or rhythmicity. In contrast, inhibiting Gi/o with pertussis toxin abolished rhythms in most neurons and desynchronized the population, phenocopying the loss of vasoactive intestinal polypeptide (VIP). Daily VIP receptor agonist treatment restored synchrony and rhythmicity to VIP−/− SCN cultures during continuous GABA receptor antagonism but not during Gi/o blockade. Pertussis toxin did not affect circadian cycling of the liver, suggesting that Gi/o plays a specialized role in maintaining SCN rhythmicity. We conclude that endogenous GABA controls the amplitude of SCN neuronal rhythms by reducing daytime firing, whereas Gi/o signaling suppresses nighttime firing, and it is necessary for synchrony among SCN neurons. We propose that Gi/o, not GABA activity, converges with VIP signaling to maintain and coordinate rhythms among SCN neurons.
Keywords: luciferase, multielectrode array, Period gene, suprachiasmatic nucleus, vasoactive intestinal polypeptide
The suprachiasmatic nuclei (SCN) of the mammalian hypothalamus serve as a master circadian pacemaker, mediating daily rhythms in behavior and physiology. SCN pacemaker function depends on near-24-h oscillations in expression of “clock genes” and in firing rate (1). Previous studies have shown that of the nearly 20,000 neurons in the bilateral SCN, one subset comprises cell-autonomous circadian clocks, and another subset requires vasoactive intestinal polypeptide (VIP) signaling to maintain daily rhythms (2).
For the SCN to coordinate coherent behavioral rhythms, SCN neurons must synchronize to one another in vivo. Blocking action potentials desynchronizes circadian rhythms among SCN neurons (3, 4). Neurotransmitters likely mediating synchrony within the SCN include VIP and GABA (2). VIP is necessary for synchrony between SCN neurons in vitro (5, 6) and for coherent behavioral rhythmicity in vivo (7, 8). GABA has been implicated because most (if not all) SCN neurons express GABA and its receptors (9–11), GABA is released in a daily rhythm within the SCN (12), and daily application of exogenous GABA synchronizes firing-rate rhythms of SCN neurons (13). How VIP mediates circadian synchrony and the necessity of GABA in this process have not been tested.
To examine the roles of endogenous GABA and G protein signaling, we recorded Period1::luciferase (Per1::luc) expression from SCN slices and firing rate and PERIOD2::luciferase (PER2::LUC) expression from individual SCN neurons. We find that long-term antagonism of GABA signaling increases the peak firing rate and precision of circadian rhythms, but surprisingly it does not impair SCN synchrony or oscillations. In contrast, inhibition of Gi/o activity with pertussis toxin (PTX) dramatically impairs coordination of daily rhythms among SCN neurons and abolishes rhythms in a subset of neurons. We conclude that GABA controls the amplitude of circadian rhythms in SCN neurons, and G protein-mediated signaling synchronizes these rhythms.
Results
PTX and Tetrodotoxin (TTX), Not GABA Receptor Antagonists, Damp SCN Ensemble Rhythms.
To assess the roles of endogenous signaling pathways on SCN rhythms and synchrony, we first screened the effects of selective antagonists on Per1::luc rhythms of cultured SCN explants. Antagonism of pathways required for SCN pacemaking or intercellular coordination would be predicted to decrease the peak-to-trough amplitude of ensemble rhythms recorded from the explant. GABAA and GABAB receptor antagonists (200 μM bicuculline and 100 μM saclofen, respectively; BIC+SAC) gradually increased Per1::luc amplitudes compared with controls; this relationship was statistically significant after 10 cycles of BIC+SAC treatment (P < 0.05; n = 6 BIC+SAC explants and 11 controls, one-way ANOVA with Scheffé post hoc test; Fig. 1). Using whole-cell recordings, we confirmed that BIC+SAC effectively blocked GABA-evoked inhibitory postsynaptic currents for at least 10 days with no homeostatic increase in GABA signaling or sensitivity to GABA [see supporting information (SI) Fig. 4]. In contrast, treating SCN slices with either the voltage-gated sodium channel blocker TTX (2 μM; n = 9) or an inhibitor of Gi/o proteins, PTX (5 nM; n = 9) significantly reduced the amplitude of Per1::luc rhythms relative to baseline. Normalized peak-to-trough amplitudes for both PTX- and TTX-treated SCN were significantly lower from the third cycle of treatment until the end of recording (P < 0.05). Importantly, normalized amplitudes recovered to control levels within 5 days after washout of both TTX and PTX. Cholera toxin, a constitutive activator of G protein subunit Gs, produced a similar rate of damping to PTX- and TTX-treated treated cultures that was not reversible (n = 3; data not shown). These results indicate that, unlike GABA, G protein signaling is necessary to maintain ensemble rhythm amplitude.
Fig. 1.
TTX and PTX, not GABA receptor antagonism, damp circadian rhythms from rat Per1::luc SCN slices. (A) Representative traces of detrended bioluminescence from individual SCN cultures in each treatment group. Bars indicate the duration of treatment. (B) Mean peak-to-trough amplitudes (± SEM) of rhythms show that TTX- and PTX-treated slices damped significantly compared with untreated and BIC+SAC-treated slices (n = at least 6 explants per point). Data were normalized to the amplitude of the last cycle before drug treatment. Lines show best-fit result of three models described in Results.
Damping of TTX- and PTX-Treated Slices Is Consistent with Damping and Desynchrony of Neuronal Rhythms.
TTX blocks spike-induced neurotransmission in the SCN, and PTX irreversibly ADP-ribosylates Gi and Go proteins, inactivating them and preventing their inhibition of adenylyl cyclase (AC). To infer how the application of these drugs damps SCN ensemble rhythms, we simulated the effects of desynchrony among neuronal oscillators, damping of rhythms in individual neurons, or both on ensemble Per1::luc rhythms. Each of these three models generated rhythms for 10,000 sinusoidal oscillators of identical amplitude with a mean period of 24.4 h (14). From the sum of all resultant sinusoids, we computed the cycle-by-cycle, peak-to-trough amplitude of the ensemble output. To simulate drug-induced desynchronization, model A randomly assigned the intrinsic period of each oscillator from a normal distribution with a variable SD and allowed the oscillators to free-run from an initially synchronized state without interaction for 10 days. Within this model increasing SD indicates reduced period synchrony among the component oscillators. To simulate drug-induced damping of individual oscillators, model B varied the proportion of damped oscillators, which had identical damping lifetimes of 24.4 h (i.e., amplitude decreased 37% with each circadian cycle). We chose this damping rate to approximate the cycle-to-cycle amplitude reduction seen in single TTX-treated SCN cells (3). This model assumed a SD of period of 0.4 h, equal to that seen for firing-rate rhythms of neurons in SCN slices in vitro (15). To allow for both desynchrony and a gradual loss of rhythmicity in subsets of SCN oscillators, model C optimally fit each data set while varying both the period SD and the percentage of damped oscillators within the population.
Of the three models, model A best fit the control and BIC+SAC data, and model C best fit PTX and TTX data (Fig. 1, SI Fig. 5, and SI Table 1). Model C predicted that PTX and TTX cause 60% of oscillators to damp while the remaining oscillators drift out of phase from one another, with a SD of period of ≈0.7 h. Consistent with these predictions, TTX-induced damping of ensemble Per1::luc rhythms was shown to result from simultaneous desynchrony and a gradual amplitude reduction in mouse SCN neurons (3). In contrast, model A predicted that control or BIC+SAC data are best explained by a population of sustained oscillators with a SD of period of 0.6 or 0.4 h, respectively.
PTX-Induced Damping of Ensemble Rhythms Is SCN-Specific.
The similarity between TTX and PTX effects on ensemble rhythms suggests that neurotransmission-regulated G protein signaling is required for maintenance of SCN rhythms. We therefore hypothesized that PTX would have little effect on peripheral tissues, which lack neurotransmission. To test this hypothesis, we applied 5 nM PTX to mouse PER2::LUC SCN and liver explants. PTX caused bioluminescence rhythms from PER2::LUC SCN (n = 4) to damp at a rate similar to that of PTX-treated rat Per1::luc SCN, but it did not change the rate of damping for PER2::LUC liver rhythms (SI Fig. 6; n = 5; P > 0.2 for all cycles). Interestingly, PTX-treated SCN damped at the same rate as liver without PTX treatment (n = 5; P > 0.05 for all cycles). We conclude that PTX reduces the amplitude of circadian rhythms specifically by blocking pathways endogenous to the SCN but not represented in the liver.
PTX, Not GABA Receptor Antagonism, Impairs SCN PER2::LUC Rhythms and Synchrony in Individual Neurons.
To test the roles of GABA and Gi/o in the SCN further, we recorded PER2::LUC rhythms from individual neurons in mouse SCN slices (Fig. 2). We found that 59% of all untreated neurons (111 of 188 from three slices) recorded over 3 days showed statistically significant circadian rhythms. GABA blockade for 8–10 days increased the percentage of rhythmic neurons to 79% (208 of 264). Consistent with predictions from Per1::luc and simulation results, individual neurons showed peak bioluminescence at similar times of day, both under control conditions and on days 8–10 of BIC+SAC treatment (P < 0.05, Rayleigh test, for all three SCN). BIC+SAC narrowed the period distribution (P < 0.01, Levene and Brown–Forsythe tests), and it shortened the mean period of PER2::LUC cycling (23.8 ± 1.8 h treated vs. 24.8 ± 2.5 h control, mean ± SD; P < 0.00005). These data suggest that GABA signaling is not required for SCN clock-gene rhythms or synchrony.
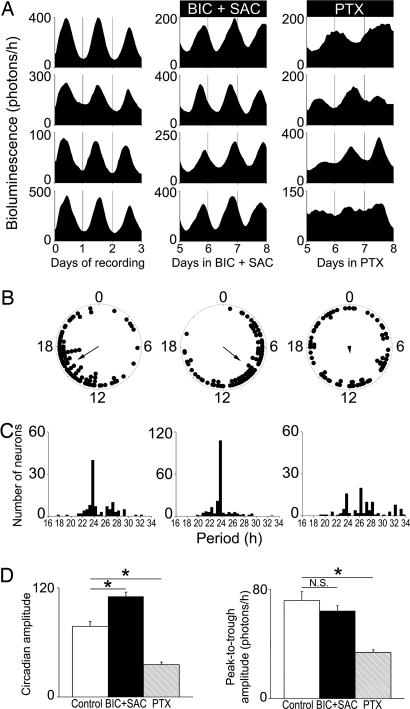
Fig. 2.
PTX, not GABA receptor antagonism, disrupts rhythms and synchrony of PER2::LUC rhythms in SCN neurons. (A) Representative PER2::LUC traces from individual neurons within mouse SCN slices under control conditions (Left), on days 5–8 of treatment with BIC+SAC (Center), and on days 5–8 of treatment with PTX (Right). (B) Representative Rayleigh plots of all rhythmic neurons within a SCN slice from each treatment group. The bioluminescence acrophase of each neuron (filled circles) and the mean phase of all neurons (arrow) are plotted for the last 24 h of each treatment. The arrow length is proportional to the magnitude of the phase clustering (r), ranging from 0 (randomly phased) to 1 (peaking at the same time). Whereas control and BIC+SAC-treated neurons maintained phase synchrony (n = 89, r = 0.63, and n = 85, r = 0.56, respectively; P < 0.001, Rayleigh test), PTX-treated neurons peaked randomly (n = 73, r = 0.16; P > 0.1). (C) Period distributions for all neurons recorded in each treatment group. PTX significantly broadened the period distribution of neurons compared with control and BIC+SAC-treated neurons (P < 0.00005, Brown–Forsythe and Levene test). (D) BIC+SAC increased, and PTX decreased the daily precision (as measured by circadian amplitude) of PER2::LUC bioluminescence rhythms in individual SCN neurons relative to controls (*, P < 0.05, ANOVA with Scheffé post hoc test). (E) Compared with controls, PTX decreased (P < 0.05), and BIC+SAC (P > 0.05) did not affect, the peak-to-trough amplitude of bioluminescence rhythms.
In contrast to BIC+SAC, long-term PTX treatment decreased the proportion of rhythmic neurons (to 45%; 106 of 235 neurons) and the synchrony between rhythmic neurons. Reduced synchrony was apparent after 8–11 days by disrupted coordination of peak bioluminescence timing among neurons within each slice (P > 0.1, Rayleigh test; n = 3) and a broadened distribution of periods (P < 0.005). PTX also significantly lengthened the mean period of PER2::LUC cycling (to 26.6 ± 3.4 h; P < 0.00001). These data, consistent with our Per1::luc and simulation results, indicate that Gi/o-mediated, not GABA, signaling is required for maintenance of clock-gene expression rhythms in SCN neurons and their synchronization.
PTX, Not GABA Receptor Antagonism, Reduces PER2::LUC Rhythm Amplitude.
To assess the effects of drug treatments on the amplitude of PER2::LUC rhythms of individual neurons, we measured the circadian and peak-to-trough amplitudes for each rhythmic neuron. Whereas changes in peak-to-trough amplitude directly reveal changes in the level of clock-gene expression throughout the day, circadian amplitude (see Materials and Methods) is a function of cycle-to-cycle precision and peak-to-trough amplitude. Precision is the inverse of cycle-to-cycle period variation (16). Remarkably, BIC+SAC increased the circadian amplitudes of neuronal PER2::LUC rhythms (110.6 ± 4.7 compared with 77.6 ± 5.6 in untreated SCN slices; P < 0.00005; Fig. 2 D and E), likely leading to the increased proportion of neurons scored as rhythmic. Importantly, BIC+SAC treatment did not increase the peak-to-trough amplitude of bioluminescence rhythms (P > 0.05), suggesting that GABA blockade increases circadian amplitude through an increase in precision of clock-gene rhythms rather than a change in expression levels.
In contrast, treatment with PTX for 8–10 days significantly decreased circadian (to 35.6 ± 2.7; P < 0.00001) and peak-to-trough amplitudes of PER2::LUC oscillations (from 71.9 ± 6.4 to 33.8 ± 2.0 photons per h; P < 0.00001), suggesting that PTX directly affects the level of clock-gene expression across the circadian cycle.
PTX, Not GABA Receptor Blockade, Impairs SCN Firing Rhythms and Synchrony.
To determine the roles of GABA and Gi/o on a functional output of individual SCN neurons, we recorded the firing patterns of neurons in high-density dispersals before and during treatment with GABA receptor antagonists or PTX. Treatment with BIC+SAC for up to 10 days did not impair the ability of SCN neurons to generate daily firing rhythms (Fig. 3; 56% rhythmic before treatment vs. 60% during; n = 96 neurons from three cultures). In contrast, PTX decreased the proportion of rhythmically firing SCN neurons [from 60% (104 of 173) before treatment to 29% (60 of 210) of neurons recorded; n = 3 cultures] after 4–5 days of treatment, similar to its effects on PER2::LUC rhythmicity.
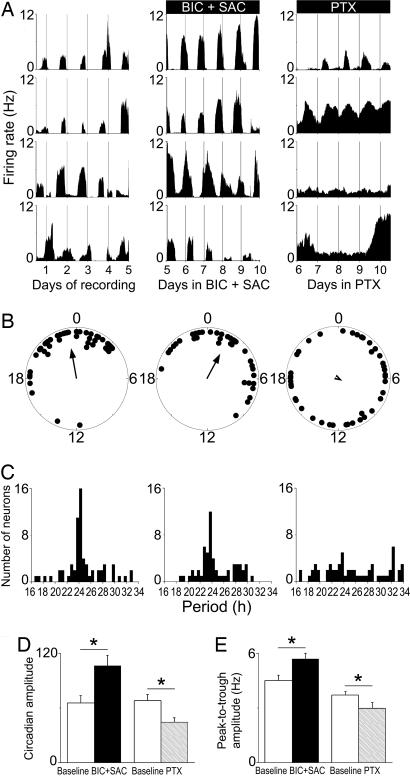
Fig. 3.
PTX, not GABA receptor antagonism, impairs firing rhythmicity and synchrony between neurons. (A) Representative firing-rate rhythms for individual SCN neurons within a control culture (Left), over the last 5 days of a 10-day BIC+SAC treatment (Center), and on the last 5 days of a 10-day PTX treatment (Right). Neurons reliably peaked at similar times during control and BIC+SAC treatment, but they were less likely to maintain rhythms, they showed lower-amplitude rhythms, and they had unstable phase relationships during PTX treatment. (B) Peak phases of all rhythmic neurons within representative SCN cultures on the last day of baseline recording (Left), on the 10th day of BIC+SAC treatment (Center), and on the 10th day of PTX treatment (Right). Rayleigh distributions of peak phases before treatment (n = 45, r = 0.66) and during BIC+SAC treatment (n = 37, r = 0.59) were statistically nonrandom (P < 0.001), but they were random during PTX treatment (n = 41, r = 0.09; P > 0.6). (C) Period distributions for all rhythmic neurons were similar before (Left) and during (Center) BIC+SAC treatment (P > 0.4), and they were significantly broadened by PTX (Right; P < 0.00001). (D) Circadian amplitudes were greater during BIC+SAC treatment than during baseline recording (*, P < 0.05) and reduced during PTX treatment relative to baseline (P < 0.05). (E) As a result of increased daily peak firing rate (P < 0.05), the peak-to-trough amplitude increased significantly during BIC+SAC treatment (P < 0.05). As a result of increased daily minimum firing rate (P < 0.00001), the peak-to-trough amplitude decreased significantly during PTX treatment (Right; P < 0.05).
Circadian synchrony was unaffected by long-term treatment with BIC+SAC. Times of peak daily firing for neurons within a culture were significantly clustered both on the last day of recording in control conditions and after 10 days of antagonist treatment (P < 0.05, Rayleigh test; n = 3 cultures). PTX, however, disrupted the coordinated timing of peak firing among neurons within a culture (P > 0.4; n = 3 cultures). Similarly, the distributions of periods for all rhythmic neurons recorded were similar during BIC+SAC treatment and under control conditions (P > 0.4), but they were significantly broadened by PTX (P < 0.00001). The mean period of firing rhythms, like PER2::LUC rhythms, lengthened in PTX (to 25.7 ± 5.0 h vs. 24.0 ± 2.8 h before treatment, mean ± SD; P < 0.01), but it did not change in BIC+SAC (24.7 ± 2.9 h during vs. 24.7 ± 3.3 h before treatment; P > 0.99). Thus, firing and gene expression patterns suggest that Gi/o signaling rather than not GABA is required to maintain rhythms and synchrony among individual SCN neurons.
GABA Blockade Increases, Whereas PTX Reduces, Firing Rate Rhythm Amplitude.
GABA receptor antagonists augmented the strength and peak-to-trough amplitudes of firing-rate rhythms (circadian amplitude: 106.5 ± 11.4 compared with baseline without antagonists 65.3 ± 8.3; P < 0.005; peak-to-trough amplitude: 5.7 ± 0.3 Hz during, 4.5 ± 0.3 Hz before treatment; P < 0.05; Fig. 3 D and E). This augmentation related to an increase in firing rates during the average daily peak of activity during BIC+SAC treatment (6.4 ± 0.4 Hz vs. 5.2 ± 0.4 Hz before treatment; P < 0.05) without a concomitant increase in firing during the daily trough of activity (0.8 ± 0.1 Hz during vs. 0.6 ± 0.1 Hz before treatment; P > 0.2). Mean firing rates were similar between baseline and BIC+SAC treatment when averaged over 4–5 days of recording (2.4 ± 0.3 Hz before treatment vs. 2.7 ± 0.3 Hz during BIC+SAC treatment; P > 0.4), which suggests that GABA receptor antagonists increase the firing rate primarily during the daily peak of firing, with little effect at other times of day. We conclude that endogenous GABA plays a critical role in regulating peak firing rates and rhythm amplitudes.
In contrast, PTX decreased the amplitudes of rhythmically firing neurons (circadian amplitude: from 68.1 ± 6.6 before treatment to 44.7 ± 4.5 during treatment, P < 0.05; peak-to-trough amplitude: 3.7 ± 0.2 Hz to 3.0 ± 0.3 Hz, P < 0.05). PTX had no effect on the peak firing rate (4.2 ± 0.2 Hz before vs. 4.6 ± 0.5 Hz during treatment; P > 0.3), but it significantly increased firing during the daily trough of activity (from 0.5 ± 0.1 Hz before treatment to 1.6 ± 0.3 Hz; P < 0.00001) and the mean firing rate (2.1 ± 0.1 Hz before treatment, vs. 2.9 ± 0.2 Hz during PTX treatment; P < 0.0005). These results indicate that chronic inhibition of Gi/o activity reduces the amplitude of firing-rate rhythms and increases overall firing by selectively interfering with the silencing of neurons during the subjective night.
Daily VIP receptor (VPAC2) Signaling Suffices for Rhythms and Synchrony in the Absence of GABA Signaling.
Because VIP signaling modulates GABA release in the SCN (12), we tested whether VIP-induced amplification and synchronization of SCN neuronal rhythms are mediated indirectly through GABA signaling. We applied VPAC2 receptor agonist Ro 25-1553 (150 nM) daily to VIP−/− SCN cultures in the continuous presence of BIC+SAC over 6 days (SI Fig. 7). Because the half-life of Ro 25-1553 activity is ≈2–4 h (17), we expect that daily applications result in 24-h rhythms of VPAC2 receptor activation. In the absence of GABAergic signaling, daily Ro 25-1553 synchronized the times of peak firing among rhythmic neurons (P < 0.05, Rayleigh test), narrowed their distribution of periods (P < 0.05), and restored the proportion of rhythmic neurons from 31% (45 of 147 neurons from four VIP−/− cultures) to 60% (88 of 147).
Daily VIP receptor (VPAC2) Signaling Is Insufficient for Rhythms and Synchrony in the Absence of Gi/o Signaling.
Because VPAC2 is thought to activate Gs rather than Gi/o in SCN neurons (12), we also tested whether daily VPAC2 activation alone could restore rhythms and synchrony to VIP−/− neurons in the absence of Gi/o function. We applied VPAC2 agonist Ro 25-1553 daily to VIP−/− SCN cultures in the continuous presence of PTX over 6 days. Daily agonist failed to resynchronize the time of peak firing among rhythmic neurons treated with PTX (P > 0.25) or their periods (25.0 ± 5.3 h before, 24.5 ± 4.6 h during, mean ± SD; P > 0.05). PTX also prevented the restoration of the proportion of rhythmic VIP−/− neurons by daily Ro 25-1553 (62 of 194 neurons or 32% from three cultures vs. 45 of 194 neurons or 23%). We conclude that in the absence of Gi/o signaling, daily VPAC2 activation is insufficient to reinstate function to VIP−/− SCN neurons.
Discussion
GABA Modulates Peak Firing Rate and Rhythm Precision, Not Synchrony, in the SCN.
Although GABA and GABA receptors are ubiquitous among SCN neurons (for review, see ref. 18) and GABA is rhythmically released in the SCN (19), its function has been controversial (for review, see ref. 20). We found that chronic GABA receptor antagonism amplified firing-rate rhythms by specifically increasing firing rates during the daily peak. In contrast, some reports have suggested that GABA can acutely excite some SCN neurons at specific circadian times (21–23). Although we cannot exclude the possibility that a minority of SCN neurons are directly or indirectly excited during GABA receptor activation, our data support the findings of others who have found an inhibitory role for GABA signaling during the day (13, 20, 24). We conclude that endogenous GABA signaling restricts the daytime peak in firing, counterbalancing an increase in membrane excitability during the day, and having little effect on the already-low nighttime firing rate of SCN neurons.
GABA plays an additional role to reduce cycle-to-cycle precision of circadian rhythms in SCN firing and clock-gene expression. Two indirect measures (circadian amplitude and period distribution of individual neurons) indicate that blockade of GABA signaling modestly improves rhythm stability. These data are consistent with observations that GABAA antagonism increases the precision of firing of SCN neurons on a millisecond time scale (25) and does not modify day–night differences in membrane potential and input resistance of SCN neurons (26). Endogenous GABA also has been shown to play a role in adjusting SCN responsiveness to photic input (27, 28), and it may play a role in coordinating entrainment of the dorsal and ventral SCN to large shifts in the light cycle (21). We conclude that GABA modulates circadian firing patterns by reducing maximal daytime firing rate, which may enhance sensitivity to depolarizing inputs.
A finding of equal or greater importance is what GABA does not do in the SCN. In contrast to its enhancement of firing-rate rhythms, GABA blockade did not affect the peak-to-trough amplitude of Period expression in individual neurons, which is evidence that a change in the amplitude of firing-rate rhythms does not necessarily coincide with a change in clock-gene expression. Furthermore, although daily application of exogenous GABA suffices to synchronize firing rhythms of dispersed SCN neurons (13), we find that it is not required for synchrony within the SCN slice. Finally, whereas the loss of VIP signaling desynchronizes circadian rhythms in the SCN (5, 6) and abolishes rhythms in GABA release (19), we found that VPAC2 agonist-induced synchrony is not mediated by GABA. Our data suggest that GABA release within the SCN does not normally produce phase shifts or affect clock-gene expression under steady-state conditions. These data strongly indicate that release of endogenous VIP rather than GABA coordinates pacemaking among SCN neurons. We conclude that intra-SCN GABA signaling is more important for entrainment of SCN neurons to environmental cycles than for their entrainment to one another.
Gi/o Proteins Mediate Rhythmicity and Synchrony in SCN Neurons.
Blocking Gi/o activity causes three changes in SCN rhythmicity, phenotypically similar to VIP or Vipr2 knockouts (6, 29): a decrease in the proportion of rhythmic neurons in the SCN from 60–70% to ≈30%, a decrease in rhythm amplitudes, and a loss of circadian synchrony between the remaining rhythmic neurons (5). Effects of PTX on PER2::LUC expression rhythms in SCN neurons also mirror circadian desynchrony and gradual damping of Per1::luc rhythms in TTX-treated SCN (3). Importantly, we find that PTX similarly affects gene expression and firing-rate rhythms by reducing the proportion of rhythmic neurons, reducing the peak-to-trough and circadian amplitudes of rhythmic neurons, abolishing the phase clustering of their daily peaks in firing, and increasing their mean and variation in period. These results suggest that PTX interferes with circadian function at both the level of the core molecular clock and circadian output. Notably, PTX had no effect on gene expression rhythms in the liver, and untreated liver rhythms damped at a rate similar to PTX-treated SCN rhythms. We conclude that Gi/o plays a specialized role in the maintenance and coordination of rhythms in the SCN, enabling SCN tissue to sustain high-amplitude rhythmicity over time.
It remains unclear whether desynchrony within the SCN leads to lower-amplitude rhythms in individual neurons or vice versa; however, these two phenomena appear to go hand in hand within the SCN network (5). The similarities between effects of VIP−/− and Vipr2−/− mutations and PTX treatment on SCN function indicate a convergent mechanism for intercellular and intracellular regulation of circadian rhythms. Consistent with this interpretation, we found that daily VPAC2 activation fails to restore circadian function to VIP−/− SCN in the presence of PTX. We and others have suggested that VIP neurotransmission could entrain and amplify rhythms within the SCN by activation of AC (2, 30). Because VPAC2 activates AC through Gs (31) and PTX-sensitive G proteins inhibit AC activity (32), we hypothesize that both daily activation and inhibition of AC are required to entrain and amplify rhythms in SCN neurons (SI Fig. 8).
In support of the proposed convergent roles of Gs and Gi/o on circadian rhythm generation and synchronization in the SCN, one fundamental difference between Vipr2−/− SCN neurons and PTX-treated SCN neurons is that, whereas Vipr2−/− neurons show a decrease in the daily peak firing rate (29), PTX increases the daily minimum firing rate in rhythmic neurons. This observation suggests that daytime activation of Gs (driven by circadian release of VIP from pacemaking neurons) and nighttime activation of Gi/o are both critical mediators of circadian rhythmicity in SCN neurons.
It is tempting to speculate on the source of Gi/o activity in the SCN. Gi/o could be regulated by extracellular or intracellular signals within the SCN. One candidate, Dexras1, is a nonreceptor-associated activator of Gi/o which inhibits AC and cAMP response element-binding protein activity in the SCN (33). The gene encoding Dexras1 is highly and rhythmically expressed in the SCN, in antiphase to the Period genes (34). Mice lacking Dexras1 show abnormal desynchronization of behavioral rhythms in constant light (35). Future studies should address which specific Gi/o activators are required for SCN synchrony and rhythmicity.
SCN Neurons Fall into Functionally Distinct Classes.
Early studies suggested that all 20,000 neurons of the bilateral SCN are cell-autonomous circadian clocks (14). More recent data suggest that blocking action potentials or VIP signaling abolishes synchrony among some SCN neurons and, critically, rhythms in the majority of cells (3, 5, 6). It is likely that SCN neurons fall into at least two functional categories: those that require intercellular communication to maintain rhythmicity, and cell-autonomous clocks that require these signals to synchronize their daily rhythms with one another. Alternatively, these treatments may weaken the pacemaking mechanism such that a random subset of cells can sustain circadian cycling. The effects of PTX are consistent with either model, and they suggest that intercellular amplification and entrainment of SCN neuronal rhythms are mediated by alternating AC stimulation and inhibition by G protein signaling.
Materials and Methods
Animals.
VIP−/− (gift from J. Waschek and C. Colwell, University of California, Los Angeles, CA), PER2::LUC knockin (gift from J. Takahashi, Northwestern University, Evanston, IL), and wild-type (C57BL/6; Charles River Laboratories, Wilmington, MA) mice and Per1::luc rats (gift from H. Tei, Mitsubishi Kagaku Institute of Life Sciences, Tokyo, Japan) were maintained as homozygous lines in a facility at Washington University.
Cell Culture.
We obtained SCN from 1- to 7-day-old mice or rats, housed in 12-h light/12-h dark cycles. Genotypes for VIP−/− and PER2::LUC mice were confirmed by PCR (8, 36). For bioluminescence recording of slices, 300-μm-thick coronal sections of bilateral SCN from Per1::luc rats or PER2::LUC mice (or 1-mm-thick slices of PER2::LUC liver) were cultured on Millicell-CM membranes (Millipore, Billerica, MA) as described in ref. 37. For recording single-neuron PER2::LUC rhythms, 150- to 300-μm-thick coronal slices of bilateral SCN were cultured on membranes for 2–6 weeks, then they were inverted onto collagen-coated glass coverslips, as described in ref. 3. Slices were then maintained in 400 μl of CO2-buffered medium supplemented with 10% newborn calf serum (Invitrogen, Carlsbad, CA) for 1–2 weeks until recording. For dispersed cultures on multielectrode arrays (MEAs; Multichannel Systems, Reutlingen, Germany), SCN were punched from 300-μm slices and dispersed by using papain (15). Viable cells from four to eight SCN were plated at >10,000 cells per mm2 on each MEA (5) and maintained in 1 ml of culture medium for 1–2 weeks before recording. After 7 days in vitro, dispersed cultures were treated with 20 μM cytosine arabinoside (Ara-C; Sigma, St. Louis, MO) to control glial proliferation.
Bioluminescence Recording.
Recordings of bioluminescence from slices of SCN or liver were made in air-buffered medium supplemented with beetle luciferin (Promega, Madison, WI) at 37°C as described in ref. 37. Single-neuron PER2::LUC bioluminescence was imaged with a Versarray 1024 cooled-CCD camera (Princeton Instruments, Trenton, NJ) from SCN slices in air-buffered medium at 37°C. Photon counts were spatially (4 × 4 pixels) and temporally (1 h) integrated by using WinView software (Princeton Instruments). Bioluminescence from individual neurons was quantified over 72–96 h of recording, and background photon counts were subtracted by using Image (National Institutes of Health, Bethesda, MD).
Multielectrode Array Recording.
We used 60-electrode MEAs (30-μm tips, 200-μm spacing) to record and discriminate neural activity at 37°C in 5% CO2 by using MC-Rack (Multichannel Systems) and Offline Sorter software (Plexon, Inc., Dallas, TX) as described in ref. 5. We plotted average firing rate per 10 min of each neuron (NeuroExplorer; Plexon, Inc.).
Drug Treatments.
GABAA antagonist BIC (200 μM; Sigma) and GABAB antagonist SAC (100 μM; Sigma) (BIC+SAC) or Bordetella PTX (5 nM; Sigma) was diluted in deionized water and stored at 4°C or −20°C. Drugs were replenished in recording medium every 3–6 days for all single-cell recordings (PER2::LUC and firing rate). Per1::luc SCN slices were treated once with BIC+SAC, PTX, or TTX (2 μM; Sigma) after a 4- to 6-day baseline recording period.
VPAC2 agonist Ro 25-1553 (38), provided by P. Robberecht (University of Brussels), was applied every 24 h for 6 days to VIP−/− SCN as described in ref. 5 in the continuous presence of BIC+SAC or PTX. BIC+SAC or PTX was applied 5 min before the initial Ro 25-1553 application, and it remained in the recording medium throughout the 6 days of agonist application without further medium changes.
Data Analysis.
All bioluminescence recordings were detrended by subtracting a 24-h running average (37). Cycle-to-cycle amplitude was measured over 4–5 days of baseline recording and then over 9–10 days after a medium exchange with or without (control) drug added. We normalized the amplitude of each cycle after the medium exchange to the amplitude of the last baseline cycle. The 2 days of recording after drug administration were not analyzed because of a transient, nonspecific increase in bioluminescence rhythm amplitudes associated with medium changes. We used χ2 periodogram (39) and fast Fourier transform–nonlinear least squares (FFT-NLLS) analyses (40) to determine independently the rhythmicity and period of firing and PER2::LUC patterns from the 3–5 days before and an equal duration during drug treatment. Periods between 16 and 32 h were considered statistically significant by χ2 periodogram if the amplitude exceeded the 99% confidence interval and by FFT-NLLS if they exceeded the 95% confidence interval. The two methods produced similar period estimates for individual neurons, differing on average by <1% for both firing rate and PER2::LUC expression rhythms. FFT-NLLS scored a larger proportion of neuron firing and gene-expression patterns as rhythmic, although the magnitude and direction of treatment effects were the same as χ2 periodogram analysis. We chose to report χ2 periodogram results for simplicity and consistency with previous reports. We measured circadian amplitude as the power above the confidence interval at the dominant period. We determined circadian synchrony within cultures by using ClockLab (Actimetrics, Wilmette, IL) to find the daily acrophase of each neuron, and we tested the resultant phase distribution for randomness with a Rayleigh test (41). We assessed differences in circadian period distributions with the Brown–Forsythe and Levene tests for equal variance.
Supplementary Material
Acknowledgments
We thank to Alexis Webb and Drs. Ute Abraham, Orie Shafer, Lou Muglia, Russ Van Gelder, and Paul Taghert for helpful discussions. This work was supported by a National Science Foundation Graduate Research Fellowship MH (to S.J.A.) and National Institutes of Health Grants MH63104 (to E.D.H.), MH073302 (to S.J.A.), and NS30888 (to J.E.H.).
Abbreviations
- AC
adenylyl cyclase
- BIC+SAC
bicuculline and saclofen
- MEA
multielectrode array
- PTX
pertussis toxin
- SCN
suprachiasmatic nuclei
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
- Vipr2
VIP receptor gene
- VPAC2
VIP receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607466103/DC1.
References
- 1.Hastings MH, Herzog ED. J Biol Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- 2.Aton SJ, Herzog ED. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 4.Honma S, Shirakawa T, Nakamura W, Honma K-I. Neurosci Lett. 2000;294:113–116. doi: 10.1016/s0304-3940(00)01558-5. [DOI] [PubMed] [Google Scholar]
- 5.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, et al. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 8.Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Am J Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamson EE, Moore RY. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 10.Moore RY, Speh JC, Leak RK. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- 11.Gao B, Fritschy JM, Moore RY. Brain Res. 1995;700:142–156. doi: 10.1016/0006-8993(95)00944-l. [DOI] [PubMed] [Google Scholar]
- 12.Itri J, Colwell CS. J Neurophysiol. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Reppert SM. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 14.Welsh DK, Logothetis DE, Meister M, Reppert SM. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 15.Herzog ED, Takahashi JS, Block GD. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 16.Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- 17.Bolin DR, Michalewsky J, Wasserman MA, O'Donnell M. Biopolymers. 1995;37:57–66. doi: 10.1002/bip.360370203. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, van den Pol AN. J Neurosci. 1998;18:1913–1922. doi: 10.1523/JNEUROSCI.18-05-01913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itri J, Michel S, Waschek JA, Colwell CS. J Neurophysiol. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gribkoff VK, Pieschl RL, Dudek FE. J Neurophysiol. 2003;90:1438–1448. doi: 10.1152/jn.01082.2002. [DOI] [PubMed] [Google Scholar]
- 21.Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Wagner S, Castel M, Gainer H, Yarom Y. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- 23.De Jeu M, Pennartz C. J Neurophysiol. 2002;87:834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- 24.Gribkoff VK, Pieschl RL, Wisialowski TA, Park WK, Strecker GJ, De Jeu MT, Pennartz CM, Dudek FE. J Biol Rhythms. 1999;14:126–130. doi: 10.1177/074873099129000515. [DOI] [PubMed] [Google Scholar]
- 25.Kononenko NI, Dudek FE. J Neurophysiol. 2004;91:267–273. doi: 10.1152/jn.00314.2003. [DOI] [PubMed] [Google Scholar]
- 26.De Jeu M, Hermes M, Pennartz C. NeuroReport. 1998;9:3725–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- 27.Gannon RL, Cato MJ, Kelley KH, Armstrong DL, Rea MA. Brain Res. 1995;694:264–270. doi: 10.1016/0006-8993(95)00854-j. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. Brain Res. 1997;759:181–189. doi: 10.1016/s0006-8993(97)00235-7. [DOI] [PubMed] [Google Scholar]
- 29.Brown TM, Hughes AT, Piggins HD. J Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao H, Zak DE, Sauter T, Schwaber J, Ogunnaike BA. Biophys J. 2006;90:1560–1571. doi: 10.1529/biophysj.105.065250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laburthe M, Couvineau A. Regul Pept. 2002;108:165–173. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 32.Albert PR, Robillard L. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 33.Graham TE, Key TA, Kilpatrick K, Dorin RI. Endocrinology. 2001;142:2631–2640. doi: 10.1210/endo.142.6.8209. [DOI] [PubMed] [Google Scholar]
- 34.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama KI, Suzuki Y, Sugano S., et al. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 35.Cheng HY, Obrietan K, Cain SW, Lee BY, Agostino PV, Joza NA, Harrington ME, Ralph MR, Penninger JM. Neuron. 2004;43:715–728. doi: 10.1016/j.neuron.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, et al. Proc Natl Acad Sci USA. 2004;101:5699–5700. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- 39.Sokolove PG, Bushell WN. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 40.Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA. J Biol Rhythms. 1997;12:204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
- 41.Batschelet E. In: Mathematics in Biology. Sibson R, Cohen JE, editors. New York: Academic; 1981. pp. 31–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.