Abstract
Net primary productivity (NPP) is enhanced under future atmospheric [CO2] in temperate forests representing a broad range of productivity. Yet questions remain in regard to how elevated [CO2]-induced NPP enhancement may be affected by climatic variations and limiting nutrient resources, as well as how this additional production is distributed among carbon (C) pools of different longevities. Using 10 years of data from the Duke free-air CO2 enrichment (Duke FACE) site, we show that spatially, the major control of NPP was nitrogen (N) availability, through its control on canopy leaf area index (L). Elevated CO2 levels resulted in greater L, and thus greater NPP. After canopy closure had occurred, elevated [CO2] did not enhance NPP at a given L, regardless of soil water availability. Additionally, using published data from three other forest FACE sites and replacing L with leaf area duration (LD) to account for differences in growing season length, we show that aboveground NPP responded to [CO2] only through the enhancement of LD. For broadleaf forests, the fraction of aboveground NPP partitioned to wood biomass saturated with increasing LD and was not enhanced by [CO2], whereas it linearly decreased for the conifer forest but was enhanced by [CO2]. These results underscore the importance of resolving [CO2] effects on L to assess the response of NPP and C allocation. Further study is necessary to elucidate the mechanisms that control the differential allocation of C among aboveground pools in different forest types.
Keywords: carbon allocation, global change, nitrogen availability, pine plantation
Current studies and modeling exercises indicate a very significant role for terrestrial ecosystems in sequestering carbon (C) and potentially mitigating increases in atmospheric CO2 concentrations ([CO2]) (1, 2), with forests contributing ≈80% of terrestrial net primary productivity (NPP) (3). Recent analysis (4) found a surprisingly consistent enhancement of NPP under elevated [CO2] across closed-canopy temperate forest ecosystems ranging greatly in productivity. This study suggested that in forests with low native canopy leaf area index (L), much of the [CO2]-induced enhancement of NPP resulted through an enhancement of L, whereas in forests with mid- to high levels of native L, most of the enhancement of NPP came through an increase in photosynthetic efficiency (4). Several questions deserve further attention: (i) How does the potential for enhancement change with other resource availability (e.g., water and nutrients), and (ii) how is the additional C gained under elevated [CO2] partitioned among C pools of differing longevities? Both questions are crucial to understanding the likely effect of elevated atmospheric [CO2] on long-term C sequestration in forests.
Ecosystem productivity shows great spatial and temporal variability. Spatial variability in productivity is most obvious among different ecosystems, resulting from differences in incoming solar radiation, temperature, precipitation, soil properties, and the species adapted to local conditions (5, 6). Variation in forest productivity can occur at much smaller scales, influenced by resource availability (7). In addition, there is often great interannual variability in global and local C sinks within terrestrial ecosystems, which has frequently been linked to climate variability (8, 9). As humans continue to alter their environment by increasing atmospheric [CO2], changing the nitrogen (N) cycle, and contributing to a changing climate, it becomes ever more critical to understand how these changes impact and can possibly be mitigated by terrestrial ecosystems.
Forests are generally expected to become greater C sinks as atmospheric CO2 levels rise (10–12). However, nutrient limitations (13–15) and increasing water deficits with climate change (16, 17) may prevent many forested regions from fully realizing dramatic increases in C sequestration. Interactions of [CO2], available nutrients, and water must be studied to predict C sinks within forests under future conditions and to inform policy and economic guidelines (18). Yet few long-term studies explore the interaction of elevated [CO2] with other growth resources on forest C processes.
Much of the spatial and temporal variability in ecosystem productivity is moderated by differences and disruptions in canopy leaf area (L). Canopy L controls light interception and thereby stand productivity (5, 19, 20). It also affects hydrological processes and thus the dynamics of soil water (21, 22) and litter production, thus the dynamics of nutrient cycling (23, 24). Maximum attainable L is primarily controlled by site quality, as defined by soil conditions, including nutrient availability, water-holding capacity and rooting volume, and long-term climate (25, 26). Many forests do not reach their genetic potential in maximum L because of water or nutrient limitations (16, 19, 27). Beyond site-imposed constraints on L, year-to-year variation in climate conditions also introduces variability in L, with species having the shortest leaf longevities being most responsive to climate variation. Climate controls not only the display of foliage (in terms of both foliage production and loss) but also the performance of L, through stomatal and biochemical limitations to photosynthesis (5). Prolonged climate-induced stresses to ecosystems are reflected in a reduction in L. Thus, L serves as an integrator, reflecting multiple constraints on site productivity (28); this is particularly useful when assessing C and energy exchanges with the atmosphere at a regional and larger scale.
Quantitative analyses of the products of photosynthesis (i.e., gross primary productivity; GPP) frequently partition C into three categories: aboveground NPP (ANPP), aboveground maintenance respiration and total belowground C allocation, which includes allocation to root production, respiration, rhizodeposition, and mychorrizal fungi. The potential C storage in an ecosystem depends on the pattern of allocation of C between and within these pools. Greater investment of C into long-lived biomass, namely wood (both aboveground and in coarse roots), yields an increase in C storage, whereas allocation to leaves and fine roots does not represent significant contributions to C storage in biomass. These tissues are short-lived and decompose quickly, although some decomposition products will be added to recalcitrant soil C pools (29). Allocation to different plant parts varies among species and has frequently been correlated to resource availability [e.g., higher allocation to roots in dry or infertile sites (30)]. Thus, site resources can impact potential C storage.
Here we use 10 years of data from the Duke free-air CO2 enrichment (FACE) site to investigate spatial and temporal causes of variability in L and NPP and in the [CO2]-induced enhancement of these factors. We expand the scope of our analysis to include recent synthesis data from three other forest FACE sites (4) to investigate the generality of findings on NPP, ANPP and L relationships across temperate forest ecosystems displaying a 5-fold range in canopy L. Finally, we explore how the fraction of ANPP partitioned to wood biomass changes with L.
Results
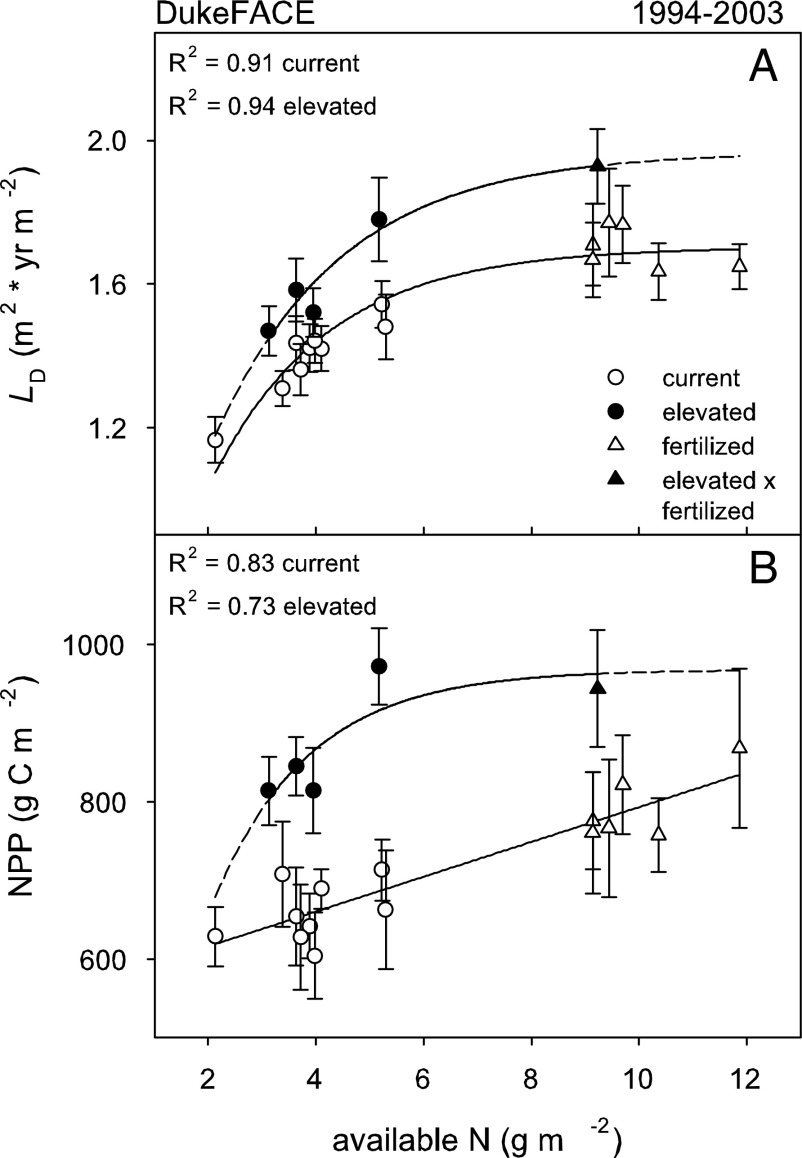
Averaged across the entire duration of the Duke FACE experiment (1994–2003), N availability exerted a strong control over LD (Fig. 1A; R2 = 0.91 and P < 0.001 for current; R2 = 0.94 and P = 0.007 for elevated), with elevated [CO2] resulting in higher LD at the same N level (P < 0.001). Note that we use one value for growing season length in each site, making the relationships with site LD and L statistically identical. The [CO2]-induced enhancement in LD was greater with increasing N; the relative enhancement in LD under elevated [CO2] was ≈13% over the entire N range, nearly doubling in absolute value from the lowest to highest N. Following from the strong dependence of LD on N, NPP was also well related to N (Fig. 1B; R2 = 0.83 and P < 0.001 for current; R2 = 0.73 and P = 0.067 for elevated). Given few replicates, analysis of covariance (ANCOVA), with [CO2] as the main effect and available N over the native range as a covariate (Fig. 1B), indicated a strong [CO2] × N interaction effect on NPP (P = 0.0525).
Fig. 1.
Five-year (fertilized plots) to 10-year (unfertilized plots) plot-level average leaf area duration (LD; m2 × yr m−2) (A) and NPP (g C m−2) (B) as functions of soil-available N under current and elevated atmospheric [CO2] in Duke FACE. Error bars indicate 1 SE among years.
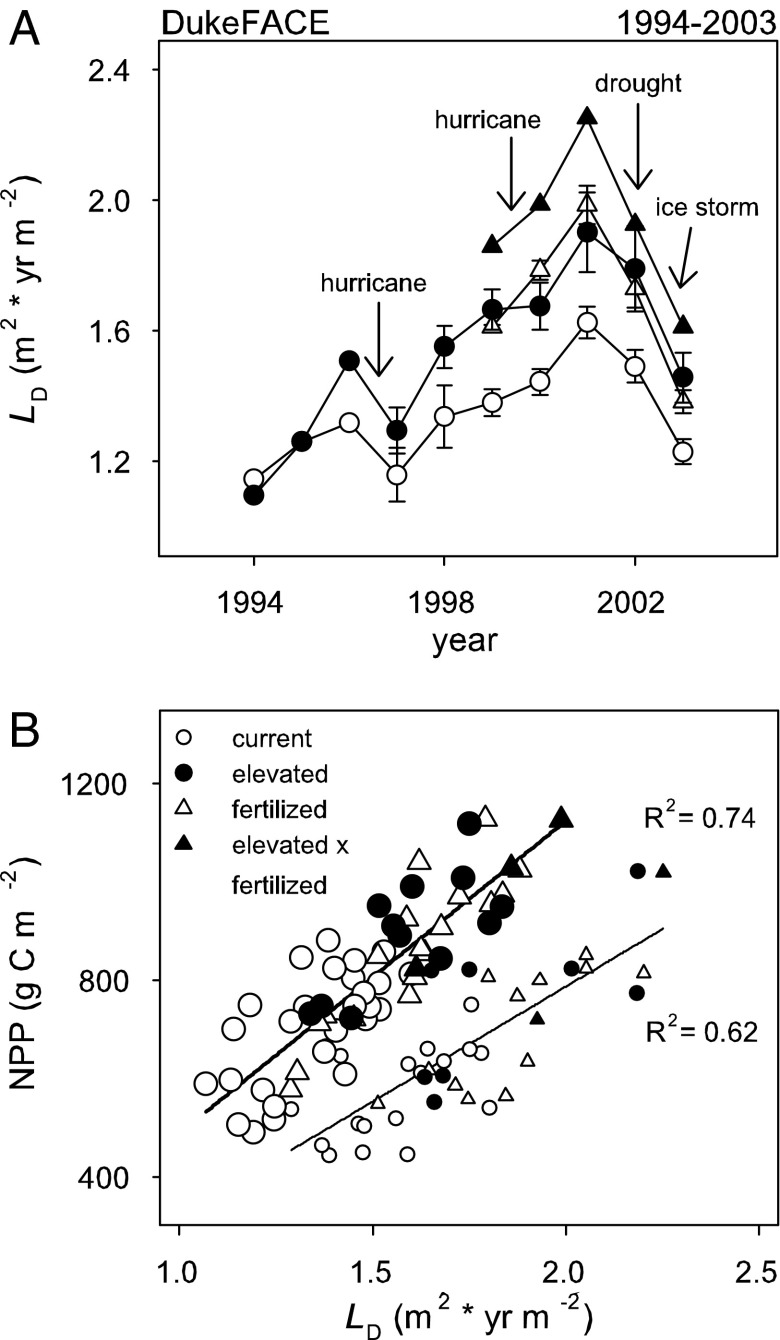
Even after accounting for N effects, plot level LD and NPP both show considerable variation within the experimental period (see error bars in Fig. 1). This variation can be attributed to a number of causes: variations in water availability, the occurrence of a severe ice storm in December 2002 resulting in canopy damage and changes in sink dynamics (31), and changes in LD as the stand progressed to a closed canopy state (Fig. 2A). Before canopy closure (through 1998), NPP at a given LD was higher than NPP after canopy closure and was unrelated to LD (P > 0.176). Averaging over the pre-canopy closure period, LD (1.2 and 1.3 for current and elevated [CO2]) was not different (P = 0.322), but NPP (693 g C m−2 at current and 873 g C m−2 at elevated [CO2]) was 26% higher under elevated [CO2] (P = 0.002). After canopy closure, NPP was linearly related to LD (Fig. 2B; P < 0.001) and was 18–21% higher at elevated [CO2] (all P < 0.016) under native soil fertility in years without severe moisture limitation (i.e., growing season precipitation minus potential evapotranspiration > 0).
Fig. 2.
Annual dynamics of leaf area duration at Duke FACE under current and elevated [CO2] (LD; m2 × yr m−2) (A) and NPP (g C m−2) as a function of LD under wet and dry conditions, as defined by growing season precipitation minus potential evapotranspiration (B). Smaller symbols in B indicate data from dry conditions; relationships were not significantly different between current and elevated [CO2] under wet or dry conditions.
Drought reduced NPP at all LD, and the negative effect of drought increased with increasing LD; the reduction in NPP was 180 g C m−2 at the lowest LD and 380 g C m−2 at the highest LD (Fig. 2B). Despite the difference in the shapes of the relationships of LD and NPP vs. N (Fig. 1), elevated [CO2] did not affect the response of NPP to LD regardless of soil moisture (P = 0.197 for the 2001–2002 dry years; P = 0.313 for wet years).
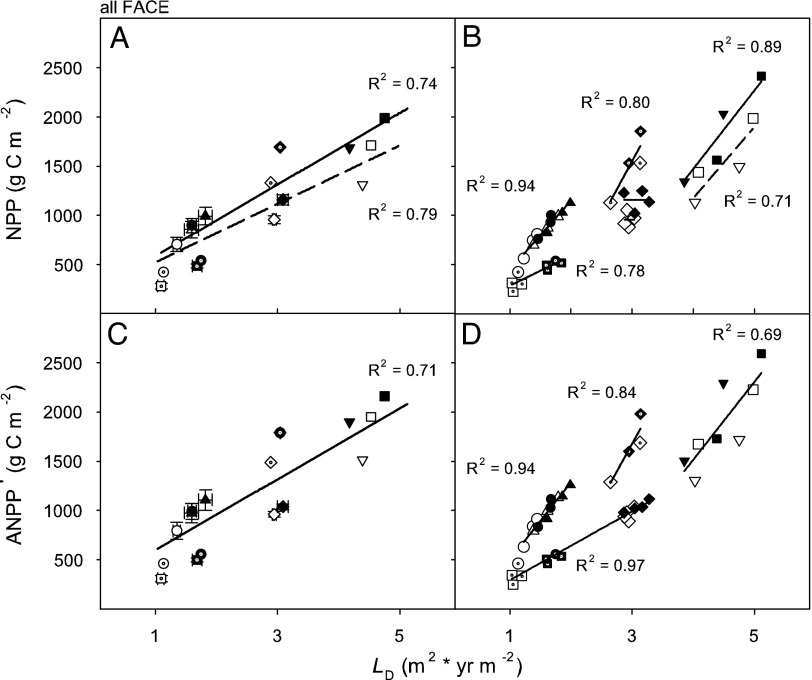
When NPP and LD data were pooled across the four FACE sites [supporting information (SI) Table 1], after excluding the data from drought years and before canopy closure at Duke FACE, NPP was linearly related to LD (Fig. 3A; n = 40, P < 0.001 for both current and elevated [CO2]; regression analysis was performed on single treatment-year data points, but we show treatment means and standard errors of each site) and significantly higher under elevated [CO2] (P = 0.039). Although the range in LD within each study is much narrower than the combined range (see standard errors in Fig. 3A), the relationship with NPP in each site could also be described by a linear fit, with the exception of Oak Ridge National Laboratory (ORNL) FACE (Fig. 3B). Differences between the [CO2] treatments in the relationship of NPP and LD were significant only for the mid- to high LD sites of ORNL-FACE (P = 0.020) and in the combination of Populus nigra and Populus alba at POP-EUROFACE (P = 0.0572, primarily because of slope difference). Similar to the relationship of NPP with LD (Fig. 3A), ANPP′ showed a linear relationship with LD (Fig. 3C with data used as in Fig. 3A; n = 40; P < 0.001). However, in contrast to the relationship with NPP, the relationship of ANPP′ with LD was similar in current and elevated [CO2] (P = 0.279), because ANPP′ in ORNL-FACE and POP-EUROFACE joined the Duke FACE and AspenFACE sites in having a single relationship with LD under both [CO2] treatments (Fig. 3D).
Fig. 3.
NPP (A and B) and ANPP′ (C and D) as functions of leaf-area duration (LD; m2 × yr m−2) at the four FACE sites. Regressions were fitted to the combined individual treatment-year data from the four sites, but treatment averages for all years within a site (with 1 SE) are shown (A and C). The treatment-year data for each site and the within-site regression fits are shown in B and D. Symbols for species: Duke FACE; P. taeda (circles for unfertilized, up triangles for fertilized), ORNL-FACE; L. styraciflua (diamonds), POP-EUROFACE; P. alba (inverted triangles), P. nigra (squares), Populus euramericana (diamonds with dot), AspenFACE; Populus tremuloides (circles with dot), P. tremuloides/Betula papyrifera (squares with dot), with filled symbols representing elevated [CO2]. For cases in which current and elevated [CO2] have different responses (in A and B), dashed and solid lines are used for these treatments, respectively.
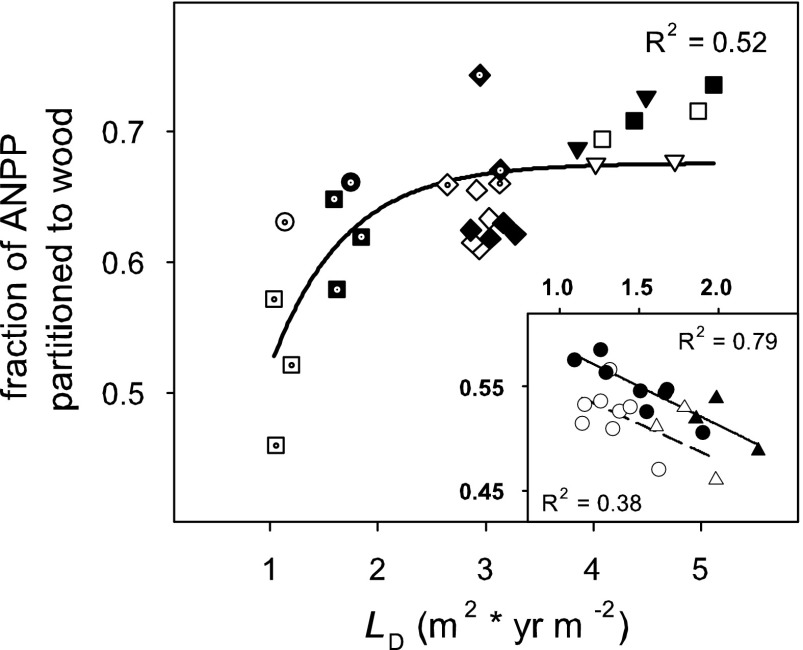
Ignoring the little C invested in the production of reproductive organs (32), we defined ANPP′ to include woody biomass and foliage production and the construction respiration associated with forming both these tissues. Across the broadleaf systems, the fraction of ANPP′ partitioned to wood production showed a saturation response with increasing LD (Fig. 4; R2 = 0.52, P < 0.001). No difference in partitioning was detected between elevated and current [CO2] treatments (P = 0.964). In contrast to the saturation seen in the broadleaf systems, the fraction of ANPP′ partitioned to wood biomass linearly declined with increasing LD in the coniferous forest at Duke FACE (Inset in Fig. 4; R2 = 0.38, P = 0.043 current [CO2]; R2 = 0.79, P < 0.001 elevated [CO2]). The difference in regression lines means that elevated [CO2] resulted in ≈3.5% more ANPP′ being partitioned to wood (P < 0.001). Data from the severe drought year of 2002 and the post-ice storm year of 2003 were not included in this analysis, because the partitioning during these years reflected recovery from disturbance and was decoupled from standing L.
Fig. 4.
The fraction of ANPP′ partitioned to wood as a function of leaf area duration (LD; m2 × yr m−2) at the four FACE sites. Duke FACE data are shown in Inset. Symbols and lines are as in Fig. 3.
Discussion
Depending on N availability, elevation of atmospheric [CO2] led to an 11–15% increase in LD in a pine forest with a hardwood component. The [CO2]-induced enhancement of NPP depended on available N, such that the NPP enhancement was largest at midrange N availabilities. This led to a different pattern of [CO2]-induced enhancement of NPP than of LD over the range of available N (Fig. 1). After canopy closure, elevated [CO2] did not enhance NPP at a given LD, under either wet or dry conditions (Fig. 2B). We also demonstrate that in a range of temperate forest ecosystems the [CO2] effect on NPP resulted from a combination of [CO2]-induced LD increase (at low LD) and increase in photosynthetic efficiency (at mid- to high LD), consistent with the previous synthesis (4). However, we discovered that the relationship between ANPP′ and L was consistently unaffected by elevated [CO2]—[CO2]-induced enhancement of ANPP′ was attributable only to a [CO2]-induced LD increase. A single ANPP′ to LD relationship for both [CO2] treatments means that any change in ANPP′ is mediated through a change in LD. This underscores the importance of LD in driving forest response to elevated [CO2]. LD integrates much of the response of aboveground productivity to [CO2] and nutrient availability in these stands. Finally, we show that partitioning of aboveground productivity to woody biomass increases and saturates with increasing LD for broadleaf stands with no apparent [CO2] effect but shows a linear decline in the coniferous forest with a clear [CO2]-induced enhancement.
The foundations of these results are familiar. Fertilization studies in the southeastern U.S. have repeatedly demonstrated that L in many Pinus taeda forests is limited by nutrient availability (commonly N, but can also be P, K, and others), and that nutrients are usually more limiting than water (19, 27). Therefore, it is not surprising that Duke FACE LD would be well related to soil N availability. Further, our results show there is an interaction of [CO2] and N in determining the increase in LD over the native and imposed (fertilized) range in N availability (Fig. 1A). It is also well known that L governs light interception and is therefore a major determinant of productivity (5, 19, 20). The increase in LD with available N results in an increase in NPP (Fig. 1B); however, the relationship of NPP and N is linear under current [CO2], whereas NPP saturated with N under elevated [CO2], resulting in an NPP enhancement that is greatest at the midrange of N availability.
Although N explains much of the spatial variability in Duke FACE NPP, a good deal of variability remains. This variation results from a number of causes, including stand development, extreme variation in water availability (with growing season averaged volumetric soil water availability ranging from 0.18 to 0.31 m3 m−3), and damage from severe ice storms (e.g., December 2002), which reduced the canopy and the aboveground C sink strength (31). Before canopy closure (in ≈1999), elevated [CO2] resulted in a greater NPP at a given LD, and LD was associated with higher NPP than at the same LD generated by disturbances after canopy closure. This may reflect a change in canopy photosynthesis as light became limiting. Drought can affect NPP by reducing production at a given L, through effects on stomatal conductance, photosynthetic rate, and sink strength, and by reducing L. In contrast to drought-induced photosynthetic reductions, drought-induced foliage loss can impact canopy-level photosynthesis over several seasons. Ice storms are associated with a traumatic reduction in L of evergreen species and extensive damage to branches where new growth would normally occur, reducing growth by both lowering L and the potential aboveground sink for carbohydrates (31, 33). We found that regardless of the cause of the variation in LD (see Fig. 2A), after canopy closure at Duke FACE both current and elevated [CO2] shared a similar NPP vs. LD relationship, which was equally shifted lower when drought reduced the efficiency of L (Fig. 2B).
The previous synthesis of data from multiple FACE sites (4), from which we drew much of our additional data (4), demonstrated that the fraction of the [CO2]-induced NPP enhancement that was attributable to increased absorption of photosynthetically active radiation declined as L under current [CO2] increased. This observation implied that most of the NPP gain in forests with low L would come through increases in canopy L, whereas in forest with mid- to high L nearly the entire NPP enhancement would result from an increase in light-use efficiency. Although our results reflect a different way of quantifying the NPP response to elevated [CO2], the outcome is largely congruent with the conclusions of the earlier work (Fig. 3A). However, focusing the analysis on ANPP′ revealed that its response to elevated [CO2] was entirely explained by the response of LD, rather than increased photosynthetic efficiency. This pattern held both within sites and among sites (Fig. 3 C and D). Expressing NPP and ANPP′ under current and future [CO2] conditions as a function of LD would facilitate assessments of elevated [CO2] effects by remote sensing.
LD explained much of the variability in the partitioning of C to wood, a moderately long-term C storage pool. For broadleaf forests, we found that the fraction of ANPP′ partitioned to wood saturated with increasing LD, and the relationship was unaffected by [CO2] (Fig. 4). Based on the observed pattern, where elevated [CO2] resulted in higher LD, the fraction of ANPP′ partitioned to wood increased; given the shape of the relationship, elevated [CO2] effects were noticeable especially at the lower range in LD. That a greater fraction of ANPP′ is partitioned to wood as LD increases is supported by previous findings that as growing conditions improve, a greater proportion of NPP is partitioned to wood (e.g., ref. 34). The saturating form of this relationship could reflect the increase in average canopy specific leaf area (SLA) that occurs as an increasing fraction of the canopy is shaded, allowing higher L for the same amount of C (e.g., refs. 35 and 36). Indeed, SLA increased 3-fold down the sweetgum canopy (37) and nearly doubled down the poplar canopies (38).
Conversely, for the pine, the fraction of ANPP′ partitioned to wood biomass decreased as LD increased (Fig. 4 Inset). This decrease of ≈10% for a near doubling of L may reflect the effect of shoot structure on the light environment within the canopy and a lesser ability of P. taeda to increase SLA. The SLA in the bottom third of the pine was only 30–50% greater than SLA in the top third (39). Moreover, unlike the finding for the broadleaf forests, elevated [CO2] enhanced the fractional allocation to wood at all levels of LD. Thus, although [CO2]-induced enhancement in LD might result in higher ANPP′ in both broadleaf and coniferous forests, changes in the fractional allocation to wood will depends on (i) how high LD is in current [CO2], and (ii) how much it will increase in elevated [CO2].
We express wood fraction in relation to ANPP′ rather than NPP, because estimating NPP, including root and mychorrhizal production and root exudates, is difficult and prone to errors, whereas ANPP is better resolved and more commonly available. Had wood partitioning been expressed instead as the fraction of NPP, it would represent a greater fraction in all sites except ORNL-FACE. This reflects the relatively high partitioning to roots observed at that site (13), as apparent from the difference in the position of the site when ANPP′ rather than NPP is assessed against LD (Fig. 3 B and D). Nevertheless, a large amount of unexplained variation remains in the partitioning of ANPP′ to wood among the broadleaf species after accounting for the effect of LD. The unexplained variation will likely persist until further studies replace the empirical correlations shown here with a mechanistic model that accounts for interaction effects of growth resources, including [CO2], on C uptake and allocation.
Combined with a companion study demonstrating that C partitioning below ground and its enhancement under elevated [CO2] are related to LD (40), the results here underscore the importance of resolving [CO2] effects on L to assess the response of C allocation in forest ecosystems. Our study suggests that the enhancement of forest C sequestration in elevated [CO2] will occur only when site resource availability allows an increase in LD and thus will depend greatly on site conditions. However, in addition to the increase in [CO2], future climate may include increased frequency of canopy disturbing events and potentially of droughts (41, 42). We show that such factors can have large effects on LD and thus C sequestration. Estimating forest C sequestration in the future must account for the combined effects of all climate change variables on C uptake and transformation.
Materials and Methods
The Duke FACE experiment is located within a loblolly pine (P. taeda L.) plantation established in 1983 on moderately low-fertility acidic clay-loam soil (Enon Series) in Orange County, NC (35°58′N, 79°08′W; elevation 163 m). The climate is warm and humid in summer and moderate in winter, with a mean annual temperature of 15.5°C. Precipitation occurs evenly throughout the year, with a long-term mean of 1,145 mm yr−1. Common broadleaf species include sweetgum (Liquidambar styraciflua) in the mid- to upper canopy and Acer rubrum, Ulmus alata, and Cornus florida in the mid- to lower canopy.
In 1993, the FACE prototype (FACEp), a 30-m diameter plot, and an adjacent untreated reference plot were established. CO2 enrichment (550 ppm during daylight hours of the growing season) began in 1994 according to the FACE protocol (43). In 1998, the FACEp and its reference plot were both split in half, and one-half of each received yearly N fertilization (11.2 g N m−2 y−1); five additional reference plot pairs (10 × 10 m) were established nearby, and one member of each pair was fertilized (14). The replicated FACE experiment (n = 3; also 30 m diameter) began CO2 enrichment (+200 ppm) in 1996. Data included in this analysis span the period 1994–2003.
Because losses of N from this system are small (44–46), available N (g N m−2 yr−1) was defined as the sum of N mineralization (measured in 1998; ref. 44), deposition (45), and fixation (46). For N-fertilized plots, it was assumed that half of the applied 11.2 g N m−2 yr−1 was available for plant uptake (47). Annual N mineralization rates for 1998 from the six FACE plots were used to develop a relationship between mineralization rates and leaf N concentrations, which was applied to 1998 leaf N concentrations taken in the FACEp plot complex to estimate N mineralization rates in these plots.
Three other FACE experiments have been conducted in forest ecosystems: ORNL-FACE in Oak Ridge, TN; AspenFACE in Rhinelander, WI; and POP-EUROFACE in Tuscania, Italy. ORNL-FACE was located in an existing L. styraciflua plantation, whereas the other sites are composed of Populus species (sometimes in combination with Acer saccharum or Betula papyrifera at AspenFACE) exposed to elevated [CO2] since stand initiation. All three are young, temperate, broadleaf forests ranging greatly in productivity and L (see ref. 4 for site characteristics). All sites are composed of plots either 25 or 30 m in diameter and use FACE technology to increase and regulate [CO2]. Detailed descriptions of site configurations and protocols are published elsewhere for ORNL-FACE (48), POP-EUROFACE (49), and AspenFACE (50).
At the Duke FACE site, total canopy L was reconstructed by using data on leaf litterfall mass and timing, SLA, and allometry for broadleaf species and needle litterfall [lagged by 2 years to account for foliage longevity (51)] and timing, combined with needle elongation rates and fascicle and shoot counts, for P. taeda. P. taeda L before 1996 was determined allometrically (52). Peak L was taken from published data for ORNL-FACE (37) and POP-EUROFACE (38, 53), whereas for AspenFACE, L available under current conditions (4) was multiplied by the [CO2]-induced enhancement of leaf biomass (54). To facilitate analysis across sites with different growing season lengths and leaf longevities (particularly for deciduous vs. evergreen), L was expressed as leaf area duration (LD; m2 × yr m−2). Because of the relative stability of growing-season L in the three broadleaf sites, LD was calculated as peak L multiplied by the fraction of the year that is considered growing season. In the Duke FACE site, the large intra-annual variation of L necessitated LD be calculated as average growing season L multiplied by the fractional length of the growing season. Further, the understory hardwood L was discounted by the ratio of pine to broadleaf SLA (g m−2) to normalize the canopy to a single species as in the other sites. The same growing season length was used for elevated and current [CO2] treatments at each site, because data from ORNL-FACE (37), POP-EUROFACE (38), and Duke FACE (55) have not shown [CO2]-induced changes in L or wood growth dynamics.
In addition to P. taeda, the analyses of NPP for Duke FACE included the production of woody (stems, branches, and roots >5 mm), foliage and fine-root biomasses for broadleaf species, most of which were in the understory. Woody biomass production was determined from the annual difference in allometrically derived standing woody biomass between consecutive years (56, 57). Foliage production for P. taeda was determined by lagging collected litterfall masses by 2 years to account for foliage longevity and, for broadleaf species foliage production, was based on same-year litterfall masses. Fine-root production was taken from published sources (58). Biomasses were converted to C by using C contents of 0.48 for aboveground P. taeda wood and foliage and wood of broadleaf species, 0.46 for broadleaf foliage, and 0.44 for all belowground biomass (14, 57). Woody biomass increment of P. taeda from fertilized plots was reduced by 8% to account for fertilization-induced density reductions (14). For the other three sites, methodology has been described (4). Briefly, NPP was calculated similarly as the sum of annual C increments (designated as Ix, where x represents specific components) and turnover (designated as Dx). Thus, NPP = Iwood + Ileaf + Icoarse root + Ifine root + Dlitterfall + Dfine root. Iwood and Icoarse root were estimated with site-specific allometry. L production (Dlitterfall) was estimated from litterfall collectors, and fine-root production was measured with minirhizotrons and in-growth cores at ORNL-FACE (13) and POP-EUROFACE (59) and calculated from published rates of aspen root turnover (60) combined with allometrically derived standing fine-root biomass at AspenFACE (54). Biomasses were converted to C with tissue-specific C contents where available; 0.5 was used as a default. Ileaf = 0 in deciduous forests. Contributions to NPP comprised by herbivory losses and roots exudates were not included, because they were not available, but herbivory losses were estimated to be small in both broadleaf and conifer species at the Duke FACE (61).
ANPP was derived from NPP by subtracting coarse and fine root production. Fine and coarse root production was taken from published values for POP-EUROFACE (62), AspenFACE (54), and ORNL-FACE (13). ANPP′ was defined as ANPP plus aboveground tissue construction respiration (where tissue construction respiration = 0.25 × ANPP; ref. 63). For the determination of the fraction of ANPP′ partitioned to wood, woody biomass production was taken from published data for POP-EUROFACE (62) and AspenFACE (54) and calculated for ORNL-FACE by subtracting published foliage biomass (37) from ANPP.
For the analyses within the Duke FACE site and the cross-FACE site analyses, the response of L, NPP, ANPP′, and the fraction of ANPP′ partitioned to woody biomass in relation to N or LD were assessed through regression analysis. Between-treatment differences were tested for differences in fit parameters as well as overall fits by using F test statistics. Curve fitting and associated statistics were done by using Proc Reg and Proc Nlin in SAS (Version 8.0; SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
This work was supported by the Office of Science (BER), U.S. Department of Energy, Grant DE-FG02-95ER62083 and by the Southern Global Change Program, Forest Service, U.S. Department of Agriculture.
Abbreviations
- NPP
net primary productivity
- GPP
gross primary productivity
- FACE
free-air CO2 enrichment
- ORNL
Oak Ridge National Laboratory
- SLA
specific leaf area.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609448103/DC1.
References
- 1.Cao M, Woodward FI. Nature. 1998;393:249–252. [Google Scholar]
- 2.Keeling CD, Chin JFS, Whorf TP. Nature. 1996;382:146–149. [Google Scholar]
- 3.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 4.Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulmans R, et al. Proc Natl Acad Sci USA. 2005;102:18052–18056. doi: 10.1073/pnas.0509478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis PG, Leverenz JW. In: Physiological Plant Ecology IV. Encyclopedia of Plant Physiol. Lange OL, editor. Vol 12D. New York: Springer; 1983. pp. 233–280. [Google Scholar]
- 6.Perry DA. Forest Ecosystems. Baltimore: John Hopkins Univ Press; 1994. [Google Scholar]
- 7.Reich PB, Grigal DF, Aber JD, Gower ST. Ecology. 1997;78:335–347. [Google Scholar]
- 8.Dai A, Fung IY. Global Biogeochem Cycles. 1993;7:599–609. [Google Scholar]
- 9.Schimel DS, Melillo J, Tian H, McGuire AD, Kicklighter D, Kittel T, Rosenbloom N, Running S, Thornton P, Ojima D, et al. Science. 2000;287:2004–2006. doi: 10.1126/science.287.5460.2004. [DOI] [PubMed] [Google Scholar]
- 10.Curtis PS, Wang X. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- 11.Körner C. Ecol App. 2000;10:1590–1619. [Google Scholar]
- 12.Norby RJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulmans R. Plant Cell Environ. 1999;22:683–714. [Google Scholar]
- 13.Norby RJ, Ledford J, Reilly CD, Miller NE, O'Neill EG. Proc Natl Acad Sci USA. 2004;101:9689–9693. doi: 10.1073/pnas.0403491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schäfer KVR, McCarthy H, Hendrey G, McNulty SG, Katul GG. Nature. 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- 15.Vitousek PM, Howarth RW. Biogeochemistry. 1991;13:87–115. [Google Scholar]
- 16.Linder S. Can J For Res. 1987;17:1157–1165. [Google Scholar]
- 17.Schulze ED, Robichaux RH, Grace J, Rundel PW, Ehleringer JR. Bioscience. 1987;37:30–37. [Google Scholar]
- 18.Houghton RA. Clim Policy. 2002;2:71–88. [Google Scholar]
- 19.Vose JM, Allen HL. For Sci. 1988;34:547–563. [Google Scholar]
- 20.Waring RH. Adv Ecol Res. 1983;13:327–354. [Google Scholar]
- 21.Oren R, Ewers BE, Todd P, Phillips N, Katul G. Ecol Appl. 1998;8:990–1002. [Google Scholar]
- 22.Stogsdill WR, Wittwer RF, Hennessey TC, Dougherty PM. For Ecol Manag. 1989;29:105–113. [Google Scholar]
- 23.Davidson EA, Belk E, Boone RD. Global Change Biol. 1998;4:217–227. [Google Scholar]
- 24.Palmroth S, Maier CA, McCarthy HR, Oishi AC, Kim H-K, Johnsen K, Katul GG, Oren R. Global Change Biol. 2005;11:421–434. [Google Scholar]
- 25.Grier CG, Running SW. Ecology. 1977;58:893–899. [Google Scholar]
- 26.Vose JM, Dougherty PM, Long JN, Smith FW, Gholz HL, Curran PJ. Ecol Bull. 1994;43:102–114. [Google Scholar]
- 27.Albaugh TJ, Allen HL, Dougherty PM, Kress LW, King JS. For Sci. 1998;44:317–328. [Google Scholar]
- 28.Landsberg JJ, Johnsen KH, Albaugh TJ, Allen HL, McKeand SE. For Sci. 2001;47:43–51. [Google Scholar]
- 29.Schlesinger WH. Annu Rev Ecol Syst. 1977;8:51–91. [Google Scholar]
- 30.Cannell MGR, Dewar RC. Adv Ecol Res. 1994;25:59–104. [Google Scholar]
- 31.McCarthy HR, Oren R, Kim H-K, Johnsen KH, Maier C, Pritchard SG, Davis MA. J Geophys Res. 2006;111:D15103. [Google Scholar]
- 32.Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J. Ecol App. 2001;11:356–370. [Google Scholar]
- 33.Wiley S, Zeide B. In: Proceedings of the Sixth Biennial Southern Silvicultural Research Conference. Coleman SS, Neary DG, editors. Asheville, NC: US Dept of Agriculture, Forest Service, Southeastern Forest Experiment Station; 1991. pp. 272–281. GTR-SE-70. [Google Scholar]
- 34.Gower ST, Gholz HL, Nakane K, Baldwin VC. Ecol Bull. 1994;43:115–135. [Google Scholar]
- 35.Oren R, Schulze ED, Matyssek R, Zimmermann R. Oecologia. 1986;70:187–193. doi: 10.1007/BF00379238. [DOI] [PubMed] [Google Scholar]
- 36.Ellsworth DS, Reich PB. Oecologia. 1993;96:169–178. doi: 10.1007/BF00317729. [DOI] [PubMed] [Google Scholar]
- 37.Norby RJ, Sholtis JD, Gunderson CA, Jawdy SS. Oecologia. 2003;136:574–584. doi: 10.1007/s00442-003-1296-2. [DOI] [PubMed] [Google Scholar]
- 38.Gielen B, Liberloo M, Bogaert J, Calfapietra C, DeAngelis P, Miglietta F, Scarascia-Mugnozza G, Ceulmans R. Global Change Biol. 2003;9:1022–1037. [Google Scholar]
- 39.DeLucia EH, George K, Hamilton JG. Tree Physiol. 2002;22:1003–1010. doi: 10.1093/treephys/22.14.1003. [DOI] [PubMed] [Google Scholar]
- 40.Palmroth S, Oren R, McCarthy HR, Johnsen KH, Finzi AC, Butnor JR, Ryan MG, Schlesinger WH. Proc Natl Acad Sci USA. 2006;103:19362–19367. doi: 10.1073/pnas.0609492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos da Silva R, Bohrer G, Werth D, Otte MJ, Avissar R. Mon Weather Rev. 2005;134:1454–1464. [Google Scholar]
- 42.Burkett V, Ritschard R, McNulty S, O'Brien JJ, Abt R, Jones J, Hatch U, Murray B, Jagtap S, Cruise J. Climate Change Impacts on the United States: The Potential Consequences of Climate Variability and Change. Cambridge, UK: Cambridge Univ Press; 2000. pp. 137–165. [Google Scholar]
- 43.Hendrey GR, Ellsworth DS, Lewin KF, Nagy J. Global Change Biol. 1999;5:293–309. [Google Scholar]
- 44.Finzi AC, DeLucia EH, Hamilton JG, Richter DD, Schlesinger WH. Oecologia. 2002;132:567–578. doi: 10.1007/s00442-002-0996-3. [DOI] [PubMed] [Google Scholar]
- 45.Finzi AC, Moore DJP, DeLucia EH, Lichter J, Hofmockel KS, Jackson RB, Kim H-S, McCarthy HR, Oren R, Pippen JS, Schlesinger WH. Ecology. 2006;87:15–25. doi: 10.1890/04-1748. [DOI] [PubMed] [Google Scholar]
- 46.Hofmockel KS, Schlesinger WH. Soil Sci Am J. 2007 in press. [Google Scholar]
- 47.Ducey M, Allen HL. For Sci. 2001;47:96–102. [Google Scholar]
- 48.Norby RJ, Todd DE, Fults J, Johnson DW. New Phytol. 2001;150:477–487. [Google Scholar]
- 49.Miglietta F, Peressotti A, Vaccari FP, Zaldei A, De Angelis P, Scarascia-Mugnozza G. New Phytol. 2001;150:465–476. [Google Scholar]
- 50.Karnosky DF, Mankovska B, Percy K, Dickson RE, Podila GK, Sober J, Noormets A, Hendrey G, Coleman MD, Kubiske M, et al. Water Air Soil Pollut. 1999;116:311–322. [Google Scholar]
- 51.Zhang S, Allen HL. Can J For Res. 1996;26:1426–1439. [Google Scholar]
- 52.Phillips N, Oren R. Ecol App. 2001;11:385–396. [Google Scholar]
- 53.Wittig VE, Bernacchi CJ, Zhu X-G, Calfapietra C, Ceulmans R, De Angelis P, Gielen B, Miglietta F, Morgan PB, Long SP. Global Change Biol. 2005;11:644–656. [Google Scholar]
- 54.King JS, Kubiske ME, Pregitzer KS, Hendrey GR, McDonald EP, Giardina CP, Quinn VS, Karnosky DF. New Phytol. 2005;168:623–636. doi: 10.1111/j.1469-8137.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 55.Moore DJP, Aref S, Ho RM, Pippen JS, Hamilton JG, DeLucia EH. Global Change Biol. 2006;12:1367–1377. [Google Scholar]
- 56.Naidu SL, DeLucia EH, Thomas RB. Can J For Res. 1998;28:1116–1124. [Google Scholar]
- 57.Schäfer KVR. PhD thesis. Durham, NC: Duke University; 2002. [Google Scholar]
- 58.Matamala R, Schlesinger WH. Global Change Biol. 2000;6:967–979. [Google Scholar]
- 59.Lukac M, Calfapietra C, Godbold DL. Global Change Biol. 2003;9:838–848. [Google Scholar]
- 60.Pregitzer KS, Zak DR, Maziasz J, DeForest J, Curtis PS, Lussenhop J. Ecol App. 2000;10:18–33. [Google Scholar]
- 61.Hamilton JG, DeLucia EH, George K, Naidu SL, Finzi AC, Schlesinger WH. Oecologia. 2002;131:250–260. doi: 10.1007/s00442-002-0884-x. [DOI] [PubMed] [Google Scholar]
- 62.Gielen B, Calfapietra C, Lukac M, Wittig VE, De Angelis P, Janssens IA, Moscatelli MC, Grego S, Cotrufo MF, Godbold DL, et al. Tree Physiol. 2005;25:1399–1408. doi: 10.1093/treephys/25.11.1399. [DOI] [PubMed] [Google Scholar]
- 63.Ryan MG. Tree Physiol. 1991;9:255–266. doi: 10.1093/treephys/9.1-2.255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.