Abstract
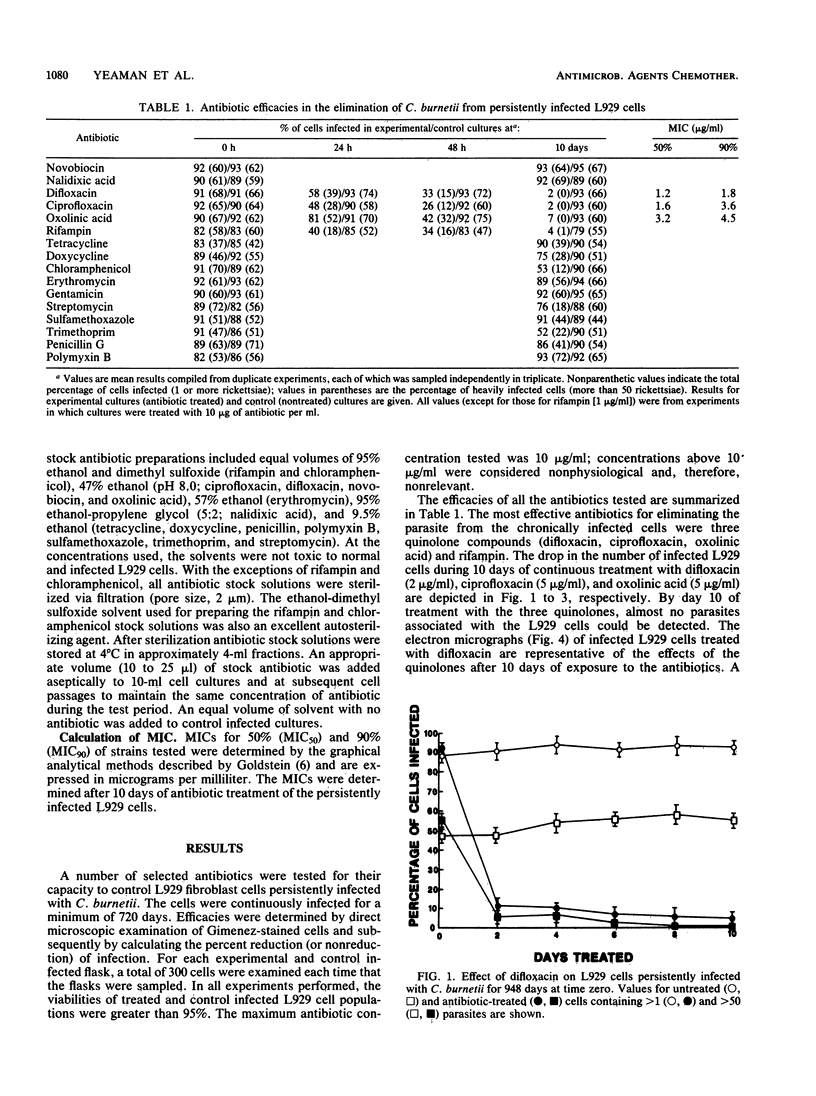
Antibiotic susceptibility testing of the rickettsial Q fever agent Coxiella burnetii was performed by using persistently infected L929 fibroblast cells. The efficacies of a variety of antibiotics with different metabolic targets were tested and compared. The most effective antibiotics in bringing about the elimination of the parasite from infected cells included several quinolone compounds and rifampin. Of the quinolone compounds tested, difloxacin (A-56619) was the most effective, followed by ciprofloxacin and oxolinic acid. These three quinolones were apparently rickettsiacidal. After 48 h of exposure to microgram amounts of the compounds (ranging from 2 micrograms of difloxacin per ml to 5 micrograms of the other two antibiotics per ml), the number of intracellular parasites markedly declined; after 10 days of treatment, very few intracellular rickettsiae were detected. Rifampin (1 microgram/ml) was also very effective in eliminating the parasites. Some of the 13 other antibiotics tested that were somewhat effective included chloramphenicol, doxycycline, and trimethoprim. The persistently infected L929 cells were found to provide a convenient system for the relatively rapid determination of the susceptibility of C. burnetii to antibiotics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akporiaye E. T., Baca O. G. Superoxide anion production and superoxide dismutase and catalase activities in Coxiella burnetii. J Bacteriol. 1983 Apr;154(1):520–523. doi: 10.1128/jb.154.1.520-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akporiaye E. T., Rowatt J. D., Aragon A. A., Baca O. G. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect Immun. 1983 Jun;40(3):1155–1162. doi: 10.1128/iai.40.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca O. G., Akporiaye E. T., Aragon A. S., Martinez I. L., Robles M. V., Warner N. L. Fate of phase I and phase II Coxiella burnetii in several macrophage-like tumor cell lines. Infect Immun. 1981 Jul;33(1):258–266. doi: 10.1128/iai.33.1.258-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca O. G., Scott T. O., Akporiaye E. T., DeBlassie R., Crissman H. A. Cell cycle distribution patterns and generation times of L929 fibroblast cells persistently infected with Coxiella burnetii. Infect Immun. 1985 Feb;47(2):366–369. doi: 10.1128/iai.47.2.366-369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., HOTTLE G. A., ROBINSON E. B. Action of streptomycin in experimental infection with Q fever. Public Health Rep. 1948 Mar 19;63(12):357–362. [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A., PARKER H., PICKENS E. G. The comparative effectiveness of aureomycin, terramycin, chloramphenicol erythromycin, and thiocymetin in suppressing experimental rickettsial infections in chick embryos. J Infect Dis. 1955 Mar-Apr;96(2):162–167. doi: 10.1093/infdis/96.2.162. [DOI] [PubMed] [Google Scholar]
- ORMSBEE R. A., PICKENS E. G. A comparison by means of the complement-fixation test of the relative potencies of chloramphenicol, aureomycin, and terramycin in experimental Q fever infections in embryonated eggs. J Immunol. 1951 Nov;67(5):437–448. [PubMed] [Google Scholar]
- Pierce M. A., Saag M. S., Dismukes W. E., Cobbs C. G. Q fever endocarditis. Am J Med Sci. 1986 Aug;292(2):104–106. doi: 10.1097/00000441-198608000-00007. [DOI] [PubMed] [Google Scholar]
- Raoult D., Etienne J., Massip P., Iaocono S., Prince M. A., Beaurain P., Benichou S., Auvergnat J. C., Mathieu P., Bachet P. Q fever endocarditis in the south of France. J Infect Dis. 1987 Mar;155(3):570–573. doi: 10.1093/infdis/155.3.570. [DOI] [PubMed] [Google Scholar]
- Raoult D., Roussellier P., Galicher V., Perez R., Tamalet J. In vitro susceptibility of Rickettsia conorii to ciprofloxacin as determined by suppressing lethality in chicken embryos and by plaque assay. Antimicrob Agents Chemother. 1986 Mar;29(3):424–425. doi: 10.1128/aac.29.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M. J., Coriz P. D., Baca O. G. A proposed model to explain persistent infection of host cells with Coxiella burnetii. J Gen Microbiol. 1986 May;132(5):1415–1422. doi: 10.1099/00221287-132-5-1415. [DOI] [PubMed] [Google Scholar]
- Tobin M. J., Cahill N., Gearty G., Maurer B., Blake S., Daly K., Hone R. Q fever endocarditis. Am J Med. 1982 Mar;72(3):396–400. doi: 10.1016/0002-9343(82)90495-8. [DOI] [PubMed] [Google Scholar]
- Varma M. P., Adgey A. A., Connolly J. H. Chronic Q fever endocarditis. Br Heart J. 1980 Jun;43(6):695–699. doi: 10.1136/hrt.43.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]



