Abstract
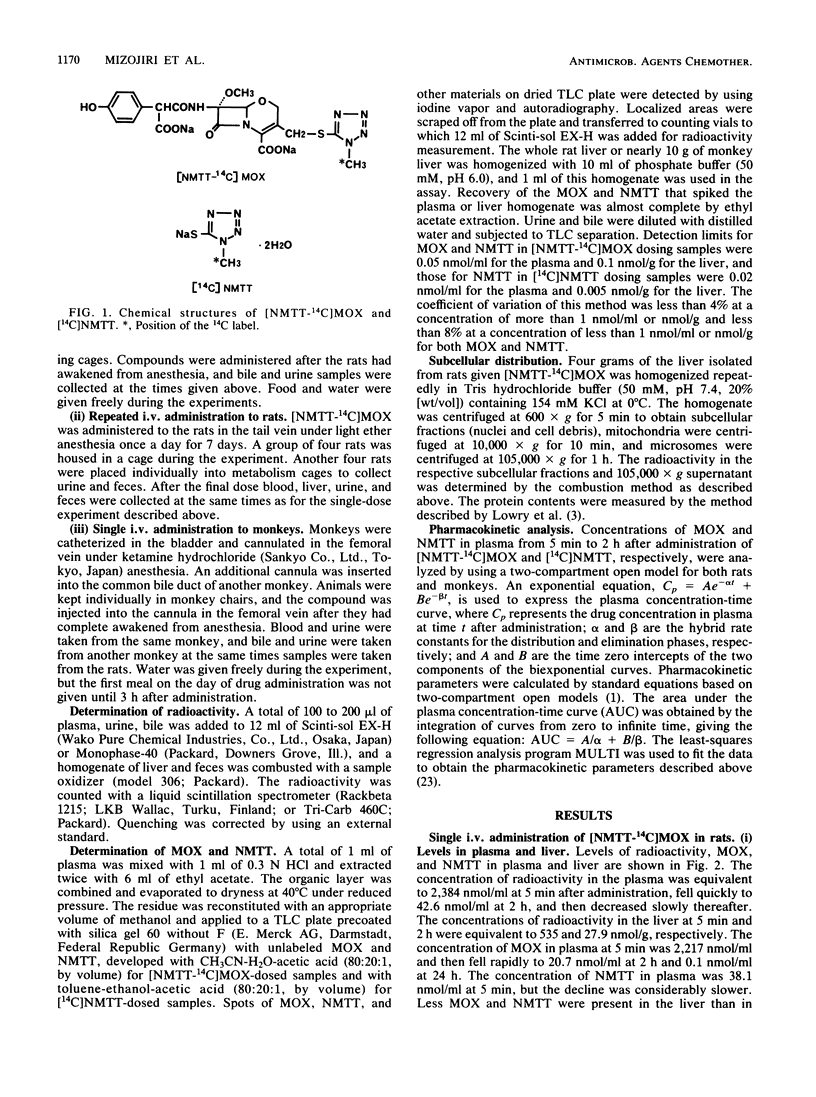
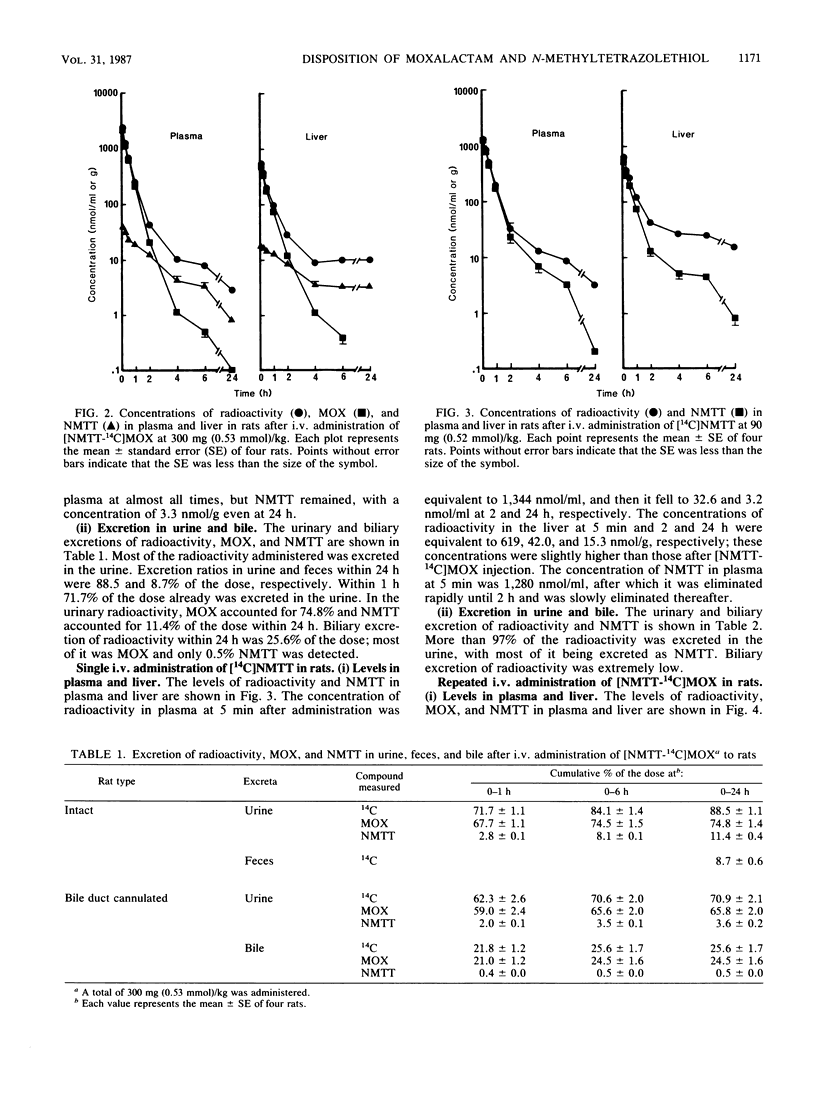
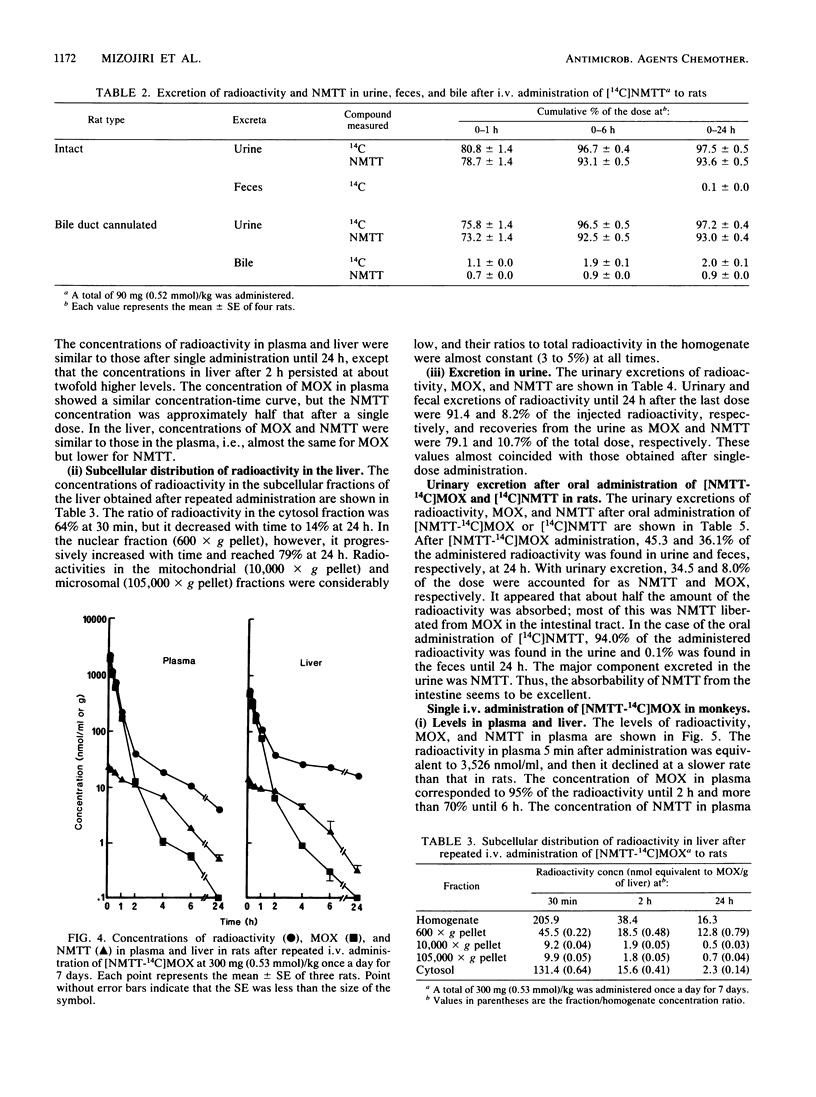
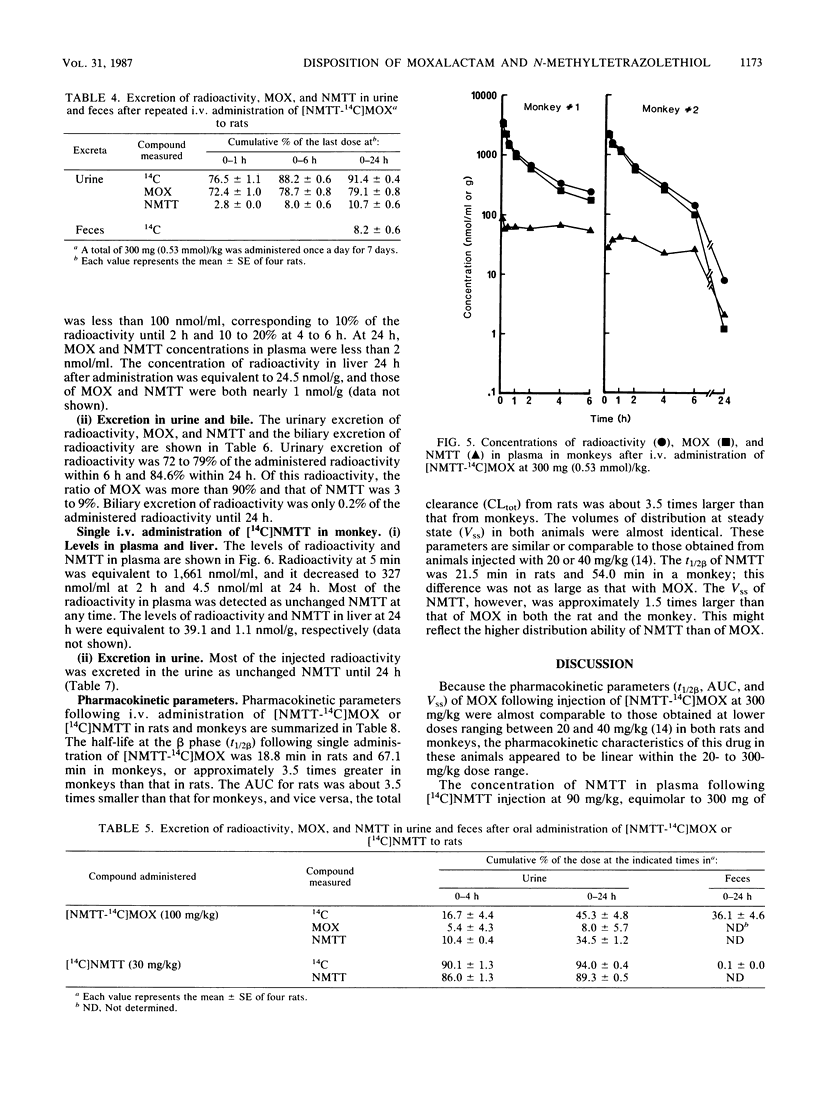
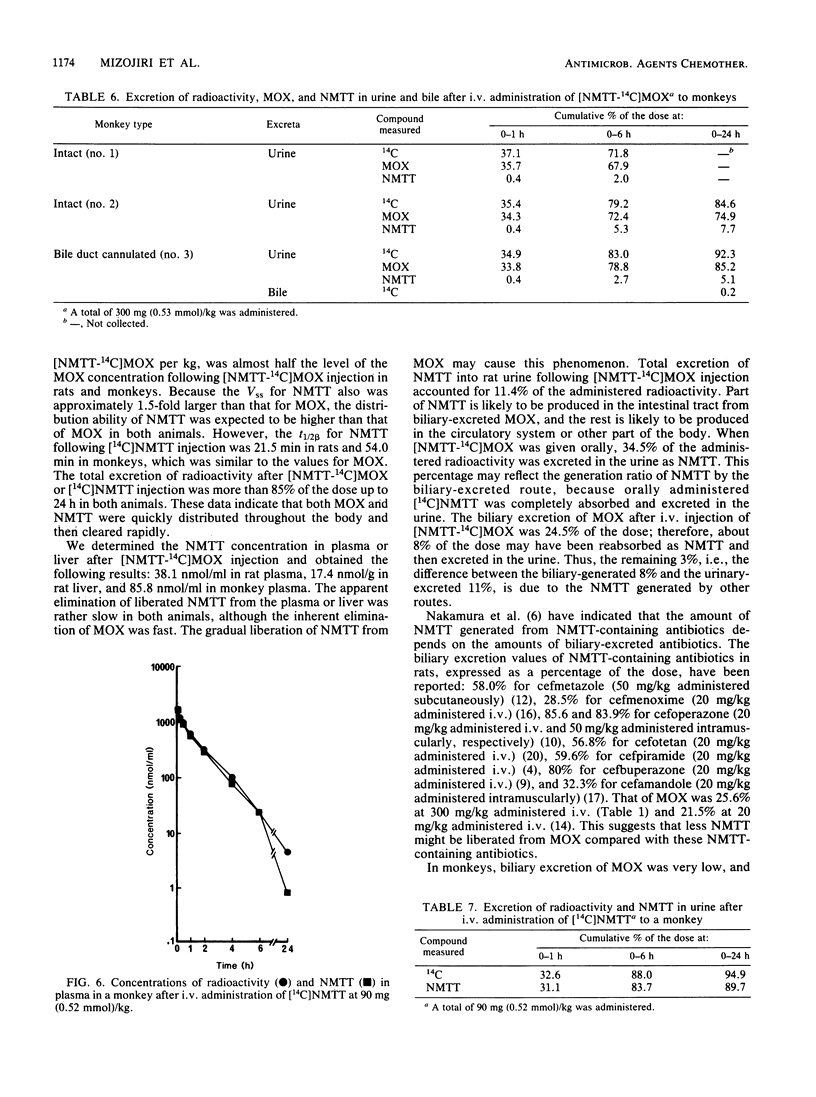
The disposition of moxalactam (MOX) and N-methyltetrazolethiol (NMTT) in rats and monkeys after intravenous injection was investigated, focusing on the in vivo liberation of NMTT, by using [NMTT-14C]MOX and [14C]NMTT. After [NMTT-14C]MOX injection, MOX levels in plasma quickly became high in both rats and monkeys and then declined, with half-lives at the beta phase of 18.8 and 67.1 min, respectively. The levels of NMTT liberated from MOX were much lower than those of MOX, but the apparent elimination was significantly slow. The levels of MOX and NMTT in rat liver were almost comparable but lower than those in plasma. With [14C]NMTT administration, the level of NMTT in plasma declined, with half-lives at the beta phase of 21.5 min in rats and 54.0 min in monkeys. After [NMTT-14C]MOX injection, most of the radioactivity was excreted in urine as MOX, with 11% of the dose in rats and 8% of the dose in monkeys eliminated as NMTT until 24 h. Total biliary excretion was 26% of the injected radioactivity in rats, and most of it was due to MOX. In one monkey, the total biliary excretion was only 0.2% of the injected radioactivity. With [14C]NMTT administration, most radioactivity was excreted in the urine as unchanged NMTT in both animals. Oral administration in rats showed that part of the biliary-excreted MOX was degraded to NMTT in the intestine and then absorbed. Repeated administration of [NMTT-14C]MOX to rats did not change the levels of MOX and NMTT in plasma or liver nor did it change the excretion profiles. Thus, accumulation of MOX and NMTT did not occur.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipsky J. J., Lewis J. C., Novick W. J., Jr Production of hypoprothrombinemia by moxalactam and 1-methyl-5-thiotetrazole in rats. Antimicrob Agents Chemother. 1984 Mar;25(3):380–381. doi: 10.1128/aac.25.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Yano K., Okuda T. Pharmacokinetics of the cephalosporin SM-1652 in mice, rats, rabbits, dogs, and rhesus monkeys. Antimicrob Agents Chemother. 1982 Aug;22(2):213–217. doi: 10.1128/aac.22.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Nakagawa A., Tanaka M., Masuda H., Hayashi Y., Saionji K. [Effects of cephem antibiotics on ethanol metabolism]. Nihon Yakurigaku Zasshi. 1984 Feb;83(2):183–191. [PubMed] [Google Scholar]
- Reddy J., Bailey R. R. Vitamin K deficiency developing in patients with renal failure treated with cephalosporin antibiotics. N Z Med J. 1980 Nov 26;92(672):378–379. [PubMed] [Google Scholar]
- Saikawa I., Nakashima Y., Sakai H., Momonoi K., Ikegami T., Minami H., Hayakawa H., Ochiai H., Todo Y. [Studies on beta-lactam antibiotics for medicinal purpose. XI. Studies on the metabolism of sodium 7-(D(-)-alpha-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-alpha-(4-hydroxyphenyl)acetamido)-3-((1-methyl-1H-tetrazole-5-yl)thiomethyl)-3-cephem-4-carboxylate (T-1551) (author's transl)]. Yakugaku Zasshi. 1980 Jun;100(6):625–640. [PubMed] [Google Scholar]
- Saikawa I., Takai A., Nakashima Y., Ikegami T., Hayakawa H., Noguchi M., Yamauchi H., Shimizu H. [Studies on absorption, distribution and excretion of 14C labelled sodium 7 beta-[(2R, 3S)-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-3-hydroxybutanamido]-7-methoxy-3-[(1-methyl-1H-tetrazol-5-YL)thiomethyl]-3-cephem-4-carboxylate (14C-T-1982) in mice and rats]. Jpn J Antibiot. 1982 Sep;35(9):2163–2173. [PubMed] [Google Scholar]
- Saikawa I., Takai A., Nakashima Y., Ikegami T., Hayakawa H., Takagi T., Yamauchi H. [Studies on the absorption distribution and excretion of 14C-labelled sodium 7-[D(-)-alpha-(4-ethyl-2,3-dioxo-1-piperazine-carboxamido)-alpha-(4-hydroxyphenyl l) acetamido]-3-[(1-methyl-1H-tetrazol-5-yl) thiomethyl]-3-cephem-4-carboxylate (14C-cefoperazone) in rats and mice]. Jpn J Antibiot. 1980 Oct;33(10):1084–1096. [PubMed] [Google Scholar]
- Shindo H., Kawai K., Ikeda T., Igarashi I., Sugawara S. Absorption, distribution, excretion and metabolism of cefmetazole in cynomolgus monkeys. J Antibiot (Tokyo) 1982 Jun;35(6):742–754. doi: 10.7164/antibiotics.35.742. [DOI] [PubMed] [Google Scholar]
- Smith G. F., Sundboom J. L. The effects of 1-methyl-5-thiotetrazole in a rat liver vitamin K-dependent carboxylase assay. Thromb Res. 1984 Mar 15;33(6):633–644. doi: 10.1016/0049-3848(84)90118-x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W., Engelke J. A., McTigue J. Effect of N-methyl-thiotetrazole on rat liver microsomal vitamin K-dependent carboxylation. Biochem Pharmacol. 1986 Jul 15;35(14):2429–2433. doi: 10.1016/0006-2952(86)90472-7. [DOI] [PubMed] [Google Scholar]
- Tanayama S., Yoshida K., Adachi K., Kondo T. Metabolic fate of SCE-1365, a new broad-spectrum cephalosporin, after parenteral administration to rats and dogs. Antimicrob Agents Chemother. 1980 Oct;18(4):511–518. doi: 10.1128/aac.18.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Ishigami T., Komeno T. Effects of latamoxef and methyltetrazolethiol on gamma-glutamylcarboxylase activity. Jpn J Pharmacol. 1984 Jul;35(3):330–333. doi: 10.1254/jjp.35.330. [DOI] [PubMed] [Google Scholar]
- Uchida K., Konishi M., Akiyoshi T., Igimi H., Asakawa S. Biliary excretion of latamoxef and N-methyltetrazolethiol in humans and rats. J Pharmacobiodyn. 1985 Nov;8(11):981–988. doi: 10.1248/bpb1978.8.981. [DOI] [PubMed] [Google Scholar]
- Weitekamp M. R., Aber R. C. Prolonged bleeding times and bleeding diathesis associated with moxalactam administration. JAMA. 1983 Jan 7;249(1):69–71. [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]


