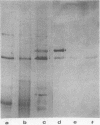
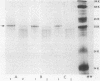
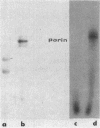
Abstract
Diminished permeation of beta-lactam antibiotics in a mutant (PCC23) of Pseudomonas aeruginosa, PAO503, was investigated. Resistance to beta-lactam antibiotics could not be correlated to a change in the beta-lactamase or target proteins in strain PCC23 but was correlated with decreased permeability. In liposome swelling assays, the permeability defect was associated with strain PCC23 porin. Amino acid analysis did not show significant difference of the porin of the mutant (PCC23) from that of the parent (PAO503). Changes in the behavior of isolated porin from PCC23 in migration in sodium dodecyl sulfate-polyacrylamide gels and in response to trypsin digestion as well as preferential labeling of PCC23 by a monoclonal antibody with a preference for the modified form of porin F (F) indicate that a structural alteration had occurred in this strain and correlated with the change in permeability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Biagi G. L., Barbaro A. M., Gamba M. F., Guerra M. C. Partition data of penicillins determined by means of reversed-phase thin-layer chromatography. J Chromatogr. 1969 May 20;41(3):371–379. doi: 10.1016/0021-9673(64)80150-3. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E. Mutation of Pseudomonas aeruginosa specifying reduced affinity for penicillin G. Antimicrob Agents Chemother. 1982 Feb;21(2):216–223. doi: 10.1128/aac.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Shahrabadi M. S., Bryan L. E. Distribution of porin and lipopolysaccharide antigens on a Pseudomonas aeruginosa permeability mutant. Antimicrob Agents Chemother. 1986 Nov;30(5):802–805. doi: 10.1128/aac.30.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Takahashi I., Nakae T. Diffusion of beta-lactam antibiotics through liposome membranes containing purified porins. Antimicrob Agents Chemother. 1982 Nov;22(5):775–780. doi: 10.1128/aac.22.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Isolation and characterization of major outer membrane proteins of Pseudomonas aeruginosa strain PAO with special reference to peptidoglycan-associated protein. J Biochem. 1979 Oct;86(4):979–989. doi: 10.1093/oxfordjournals.jbchem.a132630. [DOI] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Characterization of two surface-localized antigenic sites on porin protein F of Pseudomonas aeruginosa. Can J Microbiol. 1985 Apr;31(4):381–386. doi: 10.1139/m85-073. [DOI] [PubMed] [Google Scholar]
- Nakae R., Nakae T. Diffusion of aminoglycoside antibiotics across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 1982 Oct;22(4):554–559. doi: 10.1128/aac.22.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Ishii J. Permeability properties of Escherichia coli outer membrane containing, pore-forming proteins: comparison between lambda receptor protein and porin for saccharide permeation. J Bacteriol. 1980 Jun;142(3):735–740. doi: 10.1128/jb.142.3.735-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Parr T. R., Jr, Hancock R. E., Hanne L. F., Nicas T. I., Iglewski B. H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986 Aug;167(2):473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]