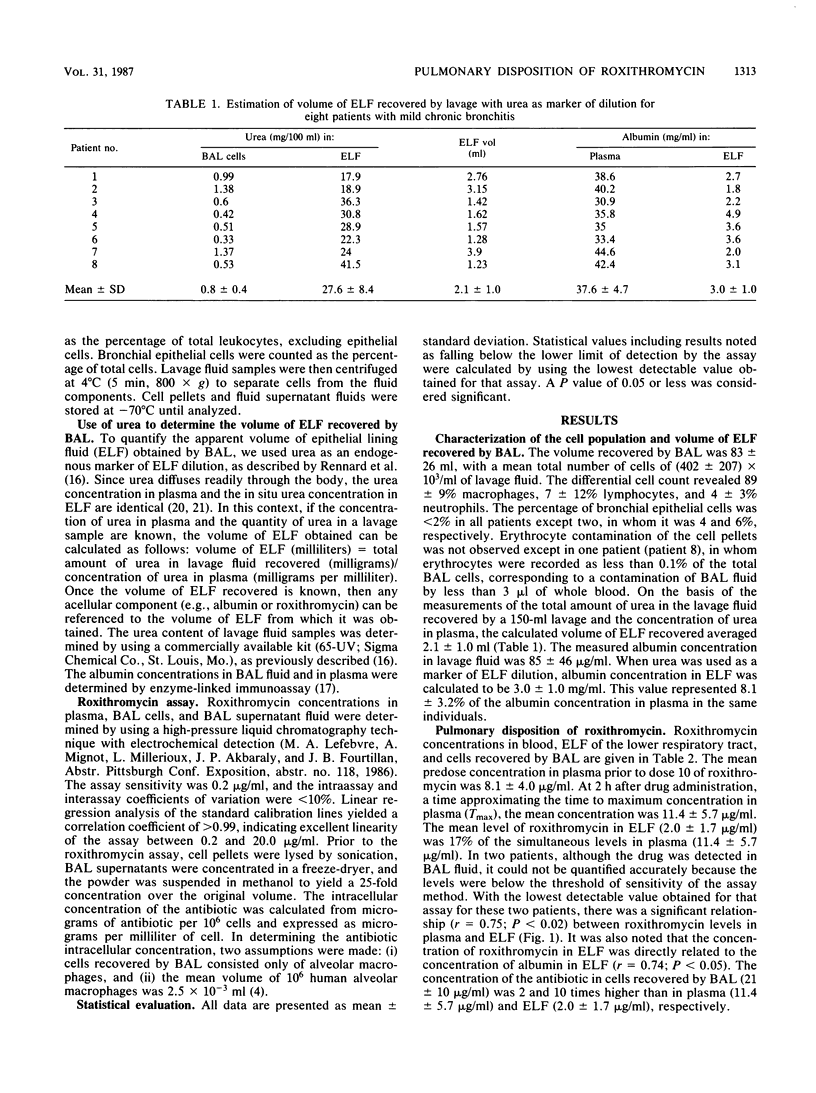
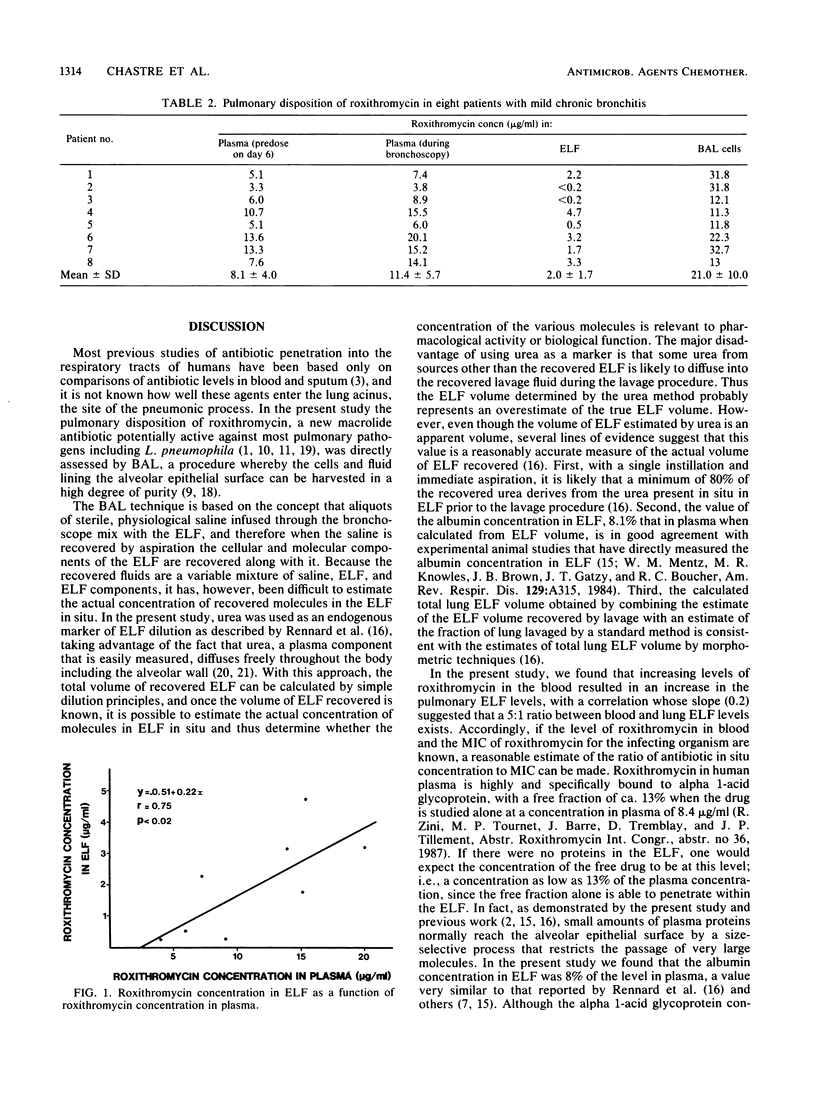
Abstract
The penetration of roxithromycin (RU 28965), an ether oxime derivative of erythromycin, into the cells and fluid lining the epithelial surface of the lower respiratory tract was studied by performing fiber-optic bronchoscopy with bronchoalveolar lavage on eight patients who had received roxithromycin at 300 mg perorally every 12 h for 5 days. The apparent volume of epithelial lining fluid recovered by bronchoalveolar lavage was determined by using urea as an endogenous marker. There was a significant relationship (r = 0.75; P less than 0.02) between roxithromycin levels in plasma and epithelial lining fluid, with a correlation whose slope suggested that the level of drug penetration into the lining fluid was 0.2. Concentrations of the antibiotic in cells recovered by bronchoalveolar lavage (21 +/- 10 micrograms/ml) were 2 and 10 times higher than in plasma (11.4 +/- 5.7 micrograms/ml) and epithelial lining fluid (2.0 +/- 1.7 micrograms/ml), respectively. Thus, when administered perorally in humans, roxithromycin is markedly accumulated by resident alveolar macrophages in concentrations largely exceeding the MBCs of the drug for most facultative intracellular pathogens including Legionella pneumophila, despite low concentrations in the epithelial lining fluid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlam T., Neu H. C. In vitro comparison of the activity of RU 28965, a new macrolide, with that of erythromycin against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Apr;25(4):529–531. doi: 10.1128/aac.25.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Gehr P., Bachofen M., Weibel E. R. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982 Aug;126(2):332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- Hand W. L., Corwin R. W., Steinberg T. H., Grossman G. D. Uptake of antibiotics by human alveolar macrophages. Am Rev Respir Dis. 1984 Jun;129(6):933–937. doi: 10.1164/arrd.1984.129.6.933. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Membrane transport of clindamycin in alveolar macrophages. Antimicrob Agents Chemother. 1982 Feb;21(2):241–247. doi: 10.1128/aac.21.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter J. F., Weiland J. E., Pacht E. R., Gadek J. E., Davis W. B. Protein permeability in the adult respiratory distress syndrome. Loss of size selectivity of the alveolar epithelium. J Clin Invest. 1986 Dec;78(6):1513–1522. doi: 10.1172/JCI112743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Intracellular multiplication of Legionnaires' disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J Clin Invest. 1983 Jan;71(1):15–26. doi: 10.1172/JCI110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. In vitro evaluation of three new macrolide antimicrobial agents, RU28965, RU29065, and RU29702, and comparisons with other orally administered drugs. Antimicrob Agents Chemother. 1983 Aug;24(2):209–215. doi: 10.1128/aac.24.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. D., Finegold S. M. Legionnaires' disease. Annu Rev Med. 1980;31:219–232. doi: 10.1146/annurev.me.31.020180.001251. [DOI] [PubMed] [Google Scholar]
- Pocidalo J. J., Albert F., Desnottes J. F., Kernbaum S. Intraphagocytic penetration of macrolides: in-vivo comparison of erythromycin and spiramycin. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):167–173. doi: 10.1093/jac/16.suppl_a.167. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenrath R. Chemical analysis of the lung alveolar surfactant obtained by alveolar micropuncture. Respir Physiol. 1973 Oct;19(1):35–46. doi: 10.1016/0034-5687(73)90088-1. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Crystal R. G. Fibronectin in human bronchopulmonary lavage fluid. Elevation in patients with interstitial lung disease. J Clin Invest. 1982 Jan;69(1):113–122. doi: 10.1172/JCI110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. E., GUYTON A. C., BISHOP V. S. PERMEABILITY OF THE ALVEOLAR MEMBRANE TO SOLUTES. Circ Res. 1965 Apr;16:353–362. doi: 10.1161/01.res.16.4.353. [DOI] [PubMed] [Google Scholar]
- Theodore J., Robin E. D., Gaudio R., Acevedo J. Transalveolar transport of large polar solutes (sucrose, inulin, and dextran). Am J Physiol. 1975 Oct;229(4):989–996. doi: 10.1152/ajplegacy.1975.229.4.989. [DOI] [PubMed] [Google Scholar]


