Abstract
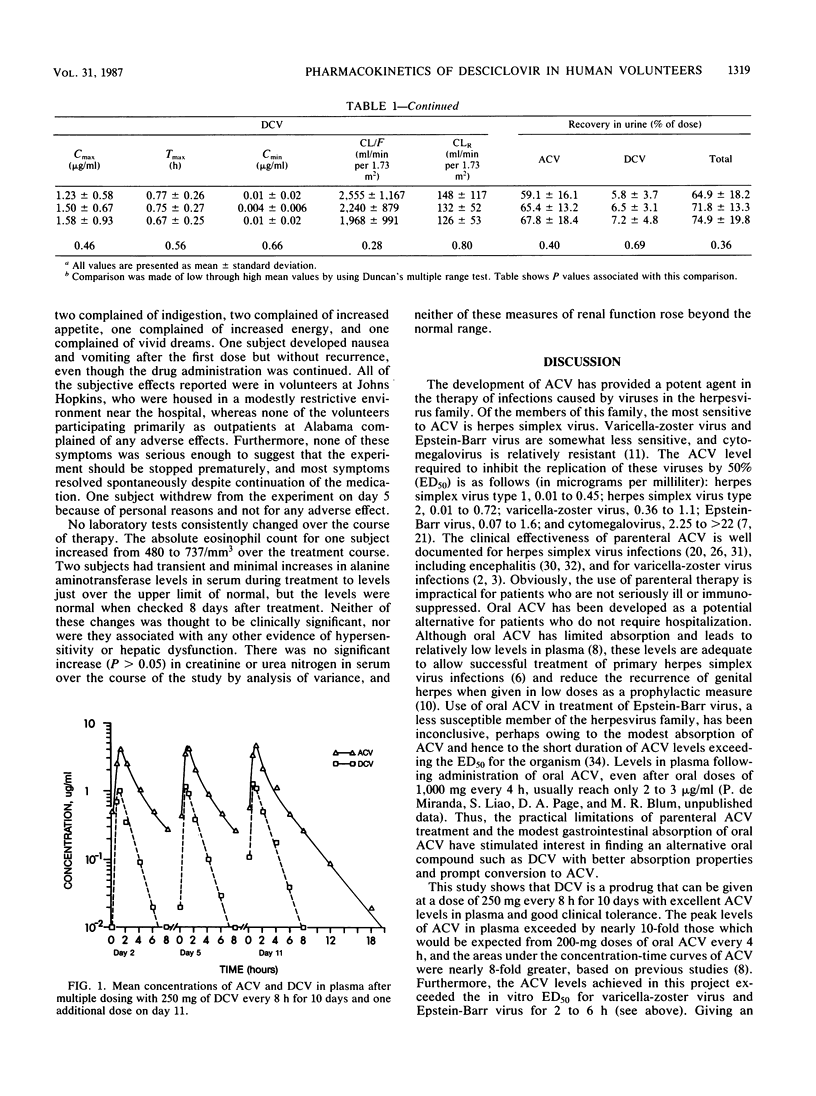
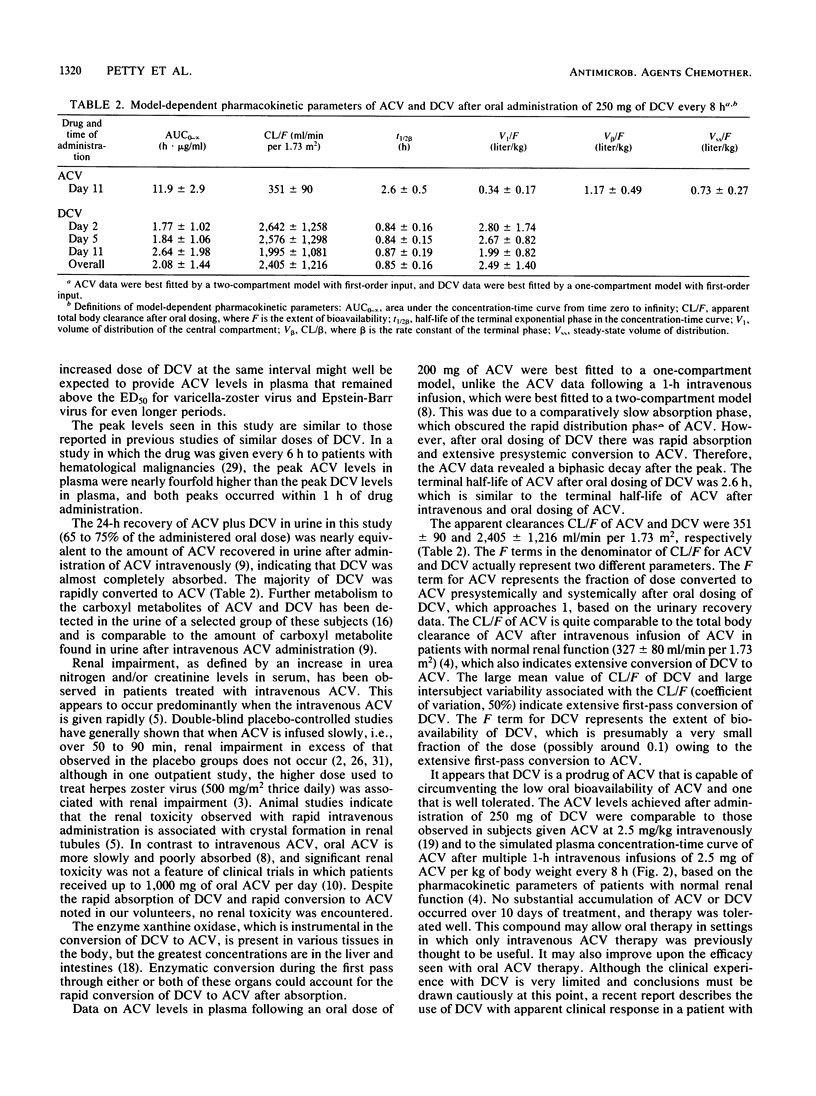
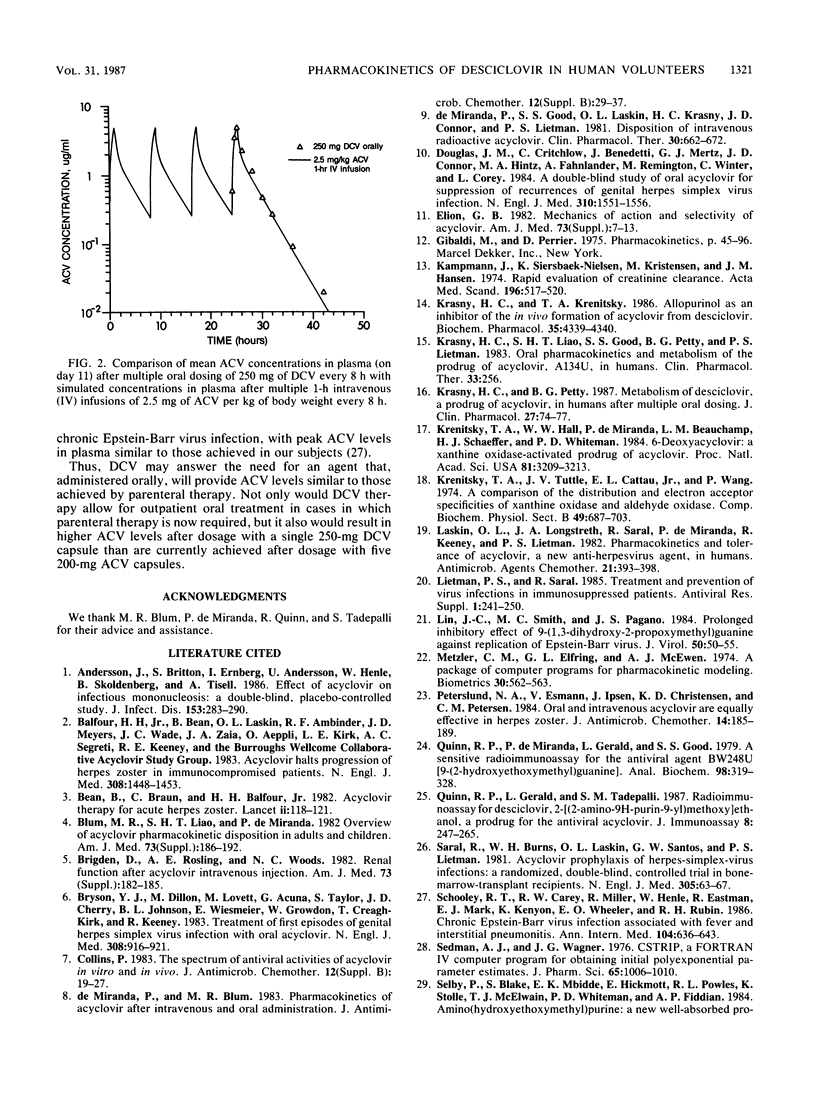
Because of the incomplete absorption of acyclovir (ACV) when given orally in humans, efforts have been made to develop a prodrug of ACV that would be better absorbed from the gastrointestinal tract and then converted in vivo to ACV. One such compound, desciclovir (DCV), is converted to acyclovir in vivo by xanthine oxidase. We gave each of 13 healthy volunteers 250 mg (about 3.25 mg/kg of body weight) of DCV orally thrice daily for 10 days, collected serial plasma and urine specimens, and measured DCV and ACV concentrations. The absorption of DCV was at least 75%, and almost two-thirds of the administered oral dose was recovered in the urine as ACV. Peak ACV levels in plasma were about 5 micrograms/ml and were reached in less than 1 h. The levels of ACV achieved in plasma were of the same magnitude as those reported for subjects given intravenous ACV at a dose of 2.5 mg/kg and approximately 10-fold higher than levels attained after administration of 200 mg of oral ACV every 4 h as measured in previous studies. The half-life of DCV was 0.85 +/- 0.16 h, compared with 2.6 +/- 0.5 h for ACV, indicating rapid conversion of DCV to ACV. There was no substantial increase in ACV levels in plasma on day 11 compared with day 2. No serious or consistent adverse effects were noted. In particular, the creatinine level in serum did not significantly rise in any subject and remained within the normal range in all.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Britton S., Ernberg I., Andersson U., Henle W., Sköldenberg B., Tisell A. Effect of acyclovir on infectious mononucleosis: a double-blind, placebo-controlled study. J Infect Dis. 1986 Feb;153(2):283–290. doi: 10.1093/infdis/153.2.283. [DOI] [PubMed] [Google Scholar]
- Balfour H. H., Jr, Bean B., Laskin O. L., Ambinder R. F., Meyers J. D., Wade J. C., Zaia J. A., Aeppli D., Kirk L. E., Segreti A. C. Acyclovir halts progression of herpes zoster in immunocompromised patients. N Engl J Med. 1983 Jun 16;308(24):1448–1453. doi: 10.1056/NEJM198306163082404. [DOI] [PubMed] [Google Scholar]
- Bean B., Braun C., Balfour H. H., Jr Acyclovir therapy for acute herpes zoster. Lancet. 1982 Jul 17;2(8290):118–121. doi: 10.1016/s0140-6736(82)91090-x. [DOI] [PubMed] [Google Scholar]
- Blum M. R., Liao S. H., de Miranda P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med. 1982 Jul 20;73(1A):186–192. doi: 10.1016/0002-9343(82)90088-2. [DOI] [PubMed] [Google Scholar]
- Brigden D., Rosling A. E., Woods N. C. Renal function after acyclovir intravenous injection. Am J Med. 1982 Jul 20;73(1A):182–185. doi: 10.1016/0002-9343(82)90087-0. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Dillon M., Lovett M., Acuna G., Taylor S., Cherry J. D., Johnson B. L., Wiesmeier E., Growdon W., Creagh-Kirk T. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med. 1983 Apr 21;308(16):916–921. doi: 10.1056/NEJM198304213081602. [DOI] [PubMed] [Google Scholar]
- Collins P. The spectrum of antiviral activities of acyclovir in vitro and in vivo. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):19–27. doi: 10.1093/jac/12.suppl_b.19. [DOI] [PubMed] [Google Scholar]
- Douglas J. M., Critchlow C., Benedetti J., Mertz G. J., Connor J. D., Hintz M. A., Fahnlander A., Remington M., Winter C., Corey L. A double-blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N Engl J Med. 1984 Jun 14;310(24):1551–1556. doi: 10.1056/NEJM198406143102402. [DOI] [PubMed] [Google Scholar]
- Elion G. B. Mechanism of action and selectivity of acyclovir. Am J Med. 1982 Jul 20;73(1A):7–13. doi: 10.1016/0002-9343(82)90055-9. [DOI] [PubMed] [Google Scholar]
- Kampmann J., Siersbaek-Nielsen K., Kristensen M., Hansen J. M. Rapid evaluation of creatinine clearance. Acta Med Scand. 1974 Dec;196(6):517–520. doi: 10.1111/j.0954-6820.1974.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Krasny H. C., Krenitsky T. A. Allopurinol as an inhibitor of the in vivo formation of acyclovir from desciclovir. Biochem Pharmacol. 1986 Dec 1;35(23):4339–4340. doi: 10.1016/0006-2952(86)90715-x. [DOI] [PubMed] [Google Scholar]
- Krasny H. C., Petty B. G. Metabolism of desciclovir, a prodrug of acyclovir, in humans after multiple oral dosing. J Clin Pharmacol. 1987 Jan;27(1):74–77. doi: 10.1177/009127008702700112. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Hall W. W., de Miranda P., Beauchamp L. M., Schaeffer H. J., Whiteman P. D. 6-Deoxyacyclovir: a xanthine oxidase-activated prodrug of acyclovir. Proc Natl Acad Sci U S A. 1984 May;81(10):3209–3213. doi: 10.1073/pnas.81.10.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Cattau E. L., Jr, Wang P. A comparison of the distribution and electron acceptor specificities of xanthine oxidase and aldehyde oxidase. Comp Biochem Physiol B. 1974 Dec 15;49(4):687–703. doi: 10.1016/0305-0491(74)90256-9. [DOI] [PubMed] [Google Scholar]
- Laskin O. L., Longstreth J. A., Saral R., de Miranda P., Keeney R., Lietman P. S. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob Agents Chemother. 1982 Mar;21(3):393–398. doi: 10.1128/aac.21.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietman P. S., Saral R. Treatment and prevention of virus infections in immunosuppressed patients. Antiviral Res. 1985;Suppl 1:241–250. doi: 10.1016/s0166-3542(85)80034-6. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein-Barr virus. J Virol. 1984 Apr;50(1):50–55. doi: 10.1128/jvi.50.1.50-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterslund N. A., Esmann V., Ipsen J., Christensen K. D., Petersen C. M. Oral and intravenous acyclovir are equally effective in herpes zoster. J Antimicrob Chemother. 1984 Aug;14(2):185–189. doi: 10.1093/jac/14.2.185. [DOI] [PubMed] [Google Scholar]
- Quinn R. P., Gerald L., Tadepalli S. Radioimmunoassay for desciclovir, 2-[(2-amino-9H-purin-9-yl)methoxy]ethanol, a prodrug for the antiviral acyclovir. J Immunoassay. 1987;8(2-3):247–265. doi: 10.1080/15321818708057025. [DOI] [PubMed] [Google Scholar]
- Quinn R. P., de Miranda P., Gerald L., Good S. S. A sensitive radioimmunoassay for the antiviral agent BW248U [9-(2-hydroxyethoxymethyl)guanine]. Anal Biochem. 1979 Oct 1;98(2):319–328. doi: 10.1016/0003-2697(79)90148-9. [DOI] [PubMed] [Google Scholar]
- Saral R., Burns W. H., Laskin O. L., Santos G. W., Lietman P. S. Acyclovir prophylaxis of herpes-simplex-virus infections. N Engl J Med. 1981 Jul 9;305(2):63–67. doi: 10.1056/NEJM198107093050202. [DOI] [PubMed] [Google Scholar]
- Schooley R. T., Carey R. W., Miller G., Henle W., Eastman R., Mark E. J., Kenyon K., Wheeler E. O., Rubin R. H. Chronic Epstein-Barr virus infection associated with fever and interstitial pneumonitis. Clinical and serologic features and response to antiviral chemotherapy. Ann Intern Med. 1986 May;104(5):636–643. doi: 10.7326/0003-4819-104-5-636. [DOI] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Sköldenberg B., Forsgren M., Alestig K., Bergström T., Burman L., Dahlqvist E., Forkman A., Frydén A., Lövgren K., Norlin K. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet. 1984 Sep 29;2(8405):707–711. doi: 10.1016/s0140-6736(84)92623-0. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Newton B., McLaren C., Flournoy N., Keeney R. E., Meyers J. D. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: a double-blind trial. Ann Intern Med. 1982 Mar;96(3):265–269. doi: 10.7326/0003-4819-96-3-265. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Alford C. A., Hirsch M. S., Schooley R. T., Luby J. P., Aoki F. Y., Hanley D., Nahmias A. J., Soong S. J. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986 Jan 16;314(3):144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- Yeh K. C., Kwan K. C. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978 Feb;6(1):79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- Yeo J. M., Fiddian A. P. Present and future of acyclovir. Scand J Infect Dis Suppl. 1985;47:165–173. [PubMed] [Google Scholar]
- de Miranda P., Blum M. R. Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Good S. S., Laskin O. L., Krasny H. C., Connor J. D., Lietman P. S. Disposition of intravenous radioactive acyclovir. Clin Pharmacol Ther. 1981 Nov;30(5):662–672. doi: 10.1038/clpt.1981.218. [DOI] [PubMed] [Google Scholar]


